Rituximab antibody | MB2A4



Rat anti Rituximab
- Product Type
- Monoclonal Antibody
- Clone
- MB2A4
- Isotype
- IgG2a
- Specificity
- Rituximab
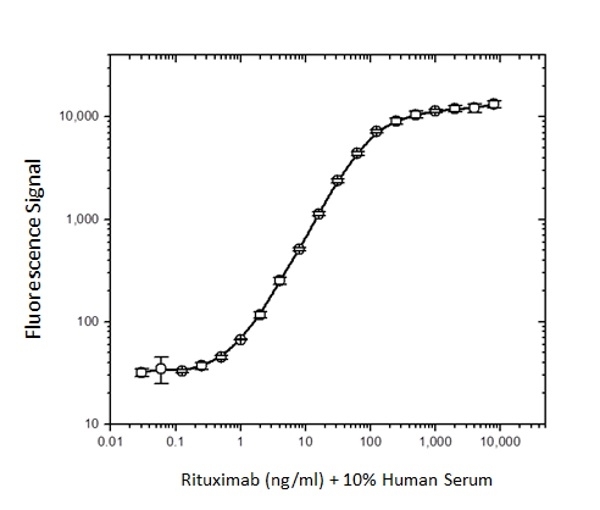
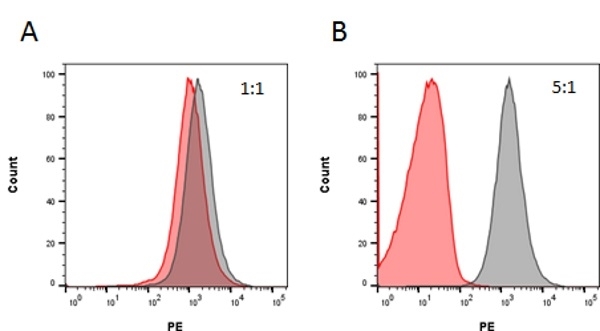
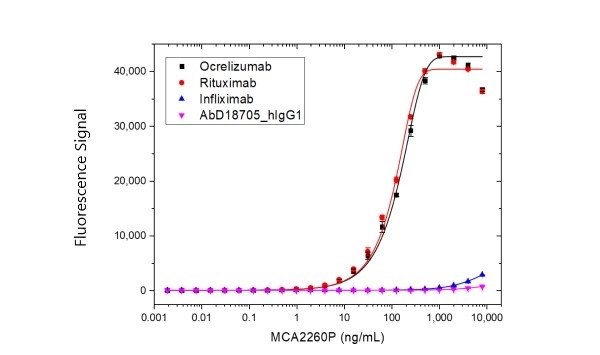
| Rat Anti-Rituximab Antibody, clone MB2A4, is an anti-idiotypic antibody that recognizes the monoclonal antibody drug rituximab. The antibody can be used to measure the levels of rituximab and biosimilar products in bioanalytical assays. Rat Anti-Rituximab Antibody also recognizes ocrelizumab, a second-generation humanized anti-CD20 antibody that binds to an epitope on CD20 that is identical or overlapping with the rituximab epitope. Clone MB2A4 has been used in ELISA to monitor the levels of rituximab in patient serum following therapy (Cragg et al. 2004 and Hampson et al. 2010). Clone MB2A4 has been used to detect rituximab bound to the surface of the Raji B cell line, however detection of rituximab bound in vivo to B-CLL cells has not been demonstrated. It is possible that complement deposition on rituximab opsonised cells inhibits binding of the Anti-Rituximab Antibody to cell bound rituximab (Beum et al. 2004). Inhibition experiments carried out with Daudi cells demonstrated that this antibody is inhibitory at a ratio of 5:1 antibody:rituximab, but does not inhibit rituximab binding to CD20 at a ratio of 1:1. Rituximab (reference product branded as Rituxan) is a chimeric mouse/human monoclonal antibody approved for the treatment of certain autoimmune diseases and cancer, including non-Hodgkin's lymphoma, chronic lymphocytic leukemia and rheumatoid arthritis. The antibody is specific for the cell surface protein CD20, which is widely expressed on B cells. Through three different mechanisms of action it eliminates B cells from the body, enabling the development of a new population of healthy B cells. View a summary of all Anti-Rituximab Antibodies. |
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- Buffer Solution
- TRIS buffered saline
- Preservative Stabilisers
- MCA2260: < 0.1% sodium azide (NaN3)
- MCA2260G: < 0.1% Sodium Azide (NaN3)
- Immunogen
- F(ab)2 fragment of Rituximab
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunised rats were fused with cells of the NS-1 mouse myeloma cell line
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
- Acknowledgements
- Rituxan is a trademark of Biogen Idec/Genentech in the USA.
MabThera is a trademark of Roche in Europe.
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA | |||
| Flow Cytometry | 50 ug/ml |
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells in 100μl
- ELISA
- Rat anti Rituximab antibody, clone MB2A4 may be used in a direct ELISA or as a detection reagent, when conjugated to HRP, in a sandwich ELISA together with HCA186 as the capture reagent. Protocol: PK bridging ELISA to measure free drug
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Rabbit F(ab')2 anti Rat IgG:FITC | STAR17B | F | 1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rabbit F(ab')2 anti Rat IgG:FITC | ||||||
| Goat F(ab')2 anti Rat IgG:FITC (Mouse Adsorbed) | STAR69 | F | 0.5 ml |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Goat F(ab')2 anti Rat IgG:FITC (Mouse Adsorbed) | ||||||
| Goat F(ab')2 anti Rat IgG:RPE (Mouse Adsorbed) | STAR73 | F | 0.5 ml |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Goat F(ab')2 anti Rat IgG:RPE (Mouse Adsorbed) | ||||||
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Human anti Rituximab | HCA061 | E | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Human anti Rituximab | ||||||
| Human anti Rituximab | HCA062 | E | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Human anti Rituximab | ||||||
| Human anti Rituximab | HCA186 | E | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Human anti Rituximab | ||||||
References for Rituximab antibody
-
Cragg, M. S. et al. (2004) A new anti-idiotype antibody capable of binding rituximab on the surface of lymphoma cells.
Blood. 104:2540-2 -
Cragg, M.S. et al. (2004) Apparent modulation of CD20 by rituximab: an alternative explanation.
Blood. 103 (10): 3989-90; author reply 3990-1. -
Pers, J.O. et al. (2007) BAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximab-treated patients with Sjögren's syndrome.
Arthritis Rheum. 56: 1464-77. -
Hampson, G. et al. (2010) Validation of an ELISA for the determination of rituximab pharmacokinetics in clinical trials subjects.
J Immunol Methods. 360 (1-2): 30-8. -
Blasco, H. et al. (2007) Evaluation of a peptide ELISA for the detection of rituximab in serum.
J Immunol Methods.325: 127-39. -
Daydé, D. et al. (2009) Tumor burden influences exposure and response to rituximab: pharmacokinetic-pharmacodynamic modeling using a syngeneic bioluminescent murine model expressing human CD20.
Blood. 113: 3765-72. -
Aung, T. et al. (2011) Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3.
Proc Natl Acad Sci U S A. 108: 15336-41. -
Schmidt, E. et al. (2009) Immunogenicity of rituximab in patients with severe pemphigus.
Clin Immunol. 132: 334-41.
View The Latest Product References
-
McDonald, V. et al. (2010) Rituximab pharmacokinetics during the management of acute idiopathic thrombotic thrombocytopenic purpura.
J Thromb Haemost. 8: 1201-8. -
Kagan, L. et al. (2012) Subcutaneous Absorption of Monoclonal Antibodies: Role of Dose, Site of Injection, and Injection Volume on Rituximab Pharmacokinetics in Rats.
Pharm Res. 29: 490-499 -
Kagan, L. and Mager, D.E. (2013) Mechanisms of subcutaneous absorption of rituximab in rats.
Drug Metab Dispos. 41: 248-55. -
Liu, X.F. et al. (2012) Validation of a Gyrolab™ assay for quantification of rituximab in human serum.
J Pharmacol Toxicol Methods. 65: 107-14. -
Kagan, L. et al. (2014) Interspecies pharmacokinetic modeling of subcutaneous absorption of rituximab in mice and rats.
Pharm Res. 31: 3265-73. -
Blasco, H. et al. (2009) Pharmacokinetics of rituximab associated with CHOP chemotherapy in B-cell non-Hodgkin lymphoma.
Fundam Clin Pharmacol. 23: 601-8. -
Illidge, T.M. et al. (2016) Short duration immunochemotherapy followed by radioimmunotherapy consolidation is effective and well tolerated in relapsed follicular lymphoma: 5-year results from a UK National Cancer Research Institute Lymphoma Group study.
Br J Haematol. 173 (2): 274-82. -
Vacher, P. et al. (2015) Localized Store-Operated Calcium Influx Represses CD95-Dependent Apoptotic Effects of Rituximab in Non-Hodgkin B Lymphomas.
J Immunol. pii: 1402942. -
Komori, M. et al. (2016) Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis.
Ann Clin Transl Neurol. 3 (3): 166-79. -
Kashiwagi, N. et al. (2017) Method for measuring anti-drug antibody
US Patent Application US20170315118A1 -
Lioger, B. et al. (2017) Antigenic burden and serum IgG concentrations influence rituximab pharmacokinetics in rheumatoid arthritis patients.
Br J Clin Pharmacol. 83 (8): 1773-81. -
Zhang, Y. et al. (2013) Stability of stock and diluted rituximab.
Am J Health Syst Pharm. 70 (5): 436-8. -
Desbois, A.C. et al. (2020) Rituximab-associated Vasculitis Flare: Incidence, Predictors, and Outcome.
J Rheumatol. 47 (6): 896-902.
- Synonyms
- Mabthera
- Rituxan
- RRID
- AB_323787
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
Always be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up