Crucial Controls and Tips For Immunohistochemistry Experiments
Optimize your experiments
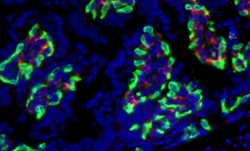
Fig. 1. IHC-frozen mouse intestine cryosections stained with anti-F4/80 (MCA497; red) and anti-PLVAP (MCA2539; green) antibodies. PVLAP stained blood vessel endothelium surrounds F4/80-labeled macrophages in the lamina propria. Nuclei were counterstained with DAPI.
As with every experimental design, the inclusion of positive and negative controls in your immunohistochemistry (IHC) staining protocol is essential. Because IHC is a rather long and complex procedure, thinking of controls early does not only save you time but also eases the troubleshooting process.
The main purpose of the listed control types is to verify that the observed antibody staining is specific.
What controls to include and why:
- Autofluorescence / endogenous tissue background staining control. As certain cell and tissue types (especially those rich in elastin, collagen and lipofuscin) display natural fluorescence it is crucial to observe samples microscopically before every staining experiment.
- Positive tissue control. Include a tissue type that expresses your protein of interest. If you do not see a staining in the positive control something has gone wrong with the staining protocol.
- Negative tissue control. Include a tissue type in which your protein of interest is not expressed. Therefore if you see staining in this type of control you know that the staining is unspecific.
- Perform a secondary antibody only control (also called no primary antibody control; follow the same staining protocol without the addition of a primary antibody) in order to ensure that the secondary antibody does not unspecifically bind to certain cellular compartments.
- Absorption controls (inhibition of staining via adsorption of the primary antibody with the purified antigen/immunogen prior to use) indicate that the primary antibody used binds exclusively to the antigen it was raised against. Although highly recommended this type of control is not routinely performed due to limitations in obtaining purified antigens/immunogens. It is also important to note that this control type only works well if the immunogen was a peptide rather than a protein.
- Isotype control. This type of control is used to ensure that the observed staining is due to the antibody binding the desired antigen and not to some general unspecific binding of the immunoglobulin to the tissue. Isotype controls are only used for controlling monoclonal antibodies and the host species and isotype of the control should match that of the primary antibody. Also isotype controls should be used at the same concentration as the primary antibody.
It is also important to block endogenous peroxidases and phosphatases prior to using alkaline phosphatase (AP) / horseradish peroxidase (HRP) antibody conjugates. For blocking endogenous peroxidase activity Bio-Rad offers ready to use peroxide blocking reagent (BUF017B).
When using avidin/biotin or streptavidin/biotin detection systems it is also important to use an avidin/biotin blocking system to minimize non-specific staining caused by endogenous avidin, biotin present in the tissue. For this specific purpose Bio-Rad offers ready to use avidin-biotin blocking system (BUF016).