MBP antibody | 12

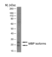


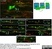

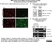


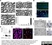



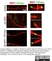




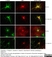
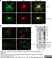
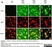




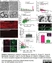




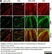

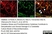
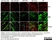

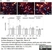

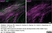


























Rat anti MBP (aa82-87)
- Product Type
- Monoclonal Antibody
- Clone
- 12
- Isotype
- IgG2a
- Specificity
- MBP
- Region
- (aa82-87)
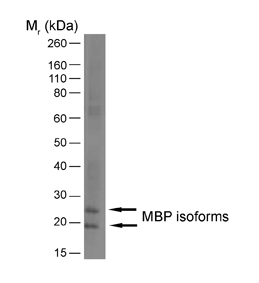
| Rat anti MBP antibody, clone 12 recognizes myelin basic protein from a wide range of species. Rat anti MBP antibody, clone 12 reacts weakly with peptides ending in the Phe 91 where the 91-92 Phe-Phe bond is broken. Synthetic peptide 82-99 reacts very well with Rat anti MBP antibody, clone 12, as does intact MBP. Further epitope analysis indicates binding to a region defined by amino acids 82-87 (DENPVV). Rat anti MBP antibody, clone 12 has been reported as being suitable for use in western blotting (Glynn et al. 1987). |
- Target Species
- Bovine
- Species Cross-Reactivity
-
Target Species Cross Reactivity Mouse Rabbit Mammals Expected from Sequence Rat Guinea Pig Sheep Human Chicken Pig - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Tissue Culture Supernatant - liquid
- Buffer Solution
- 0.1M TRIS
- Preservative Stabilisers
0.1% Sodium Azide - Immunogen
- Bovine MBP.
- Fusion Partners
- Spleen cells from an immunized outbred rat were fused with cells of the mouse NS0 myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA | |||
| Immunofluorescence | |||
| Radioimmunoassays | |||
| Western Blotting |
References for MBP antibody
-
Groome, N.P. et al. (1986) Region-specific immunoassays for human myelin basic protein.
J Neuroimmunol. 12 (4): 253-64. -
Glynn, P. et al. (1987) Basic protein dissociating from myelin membranes at physiological ionic strength and pH is cleaved into three major fragments.
J Neurochem. 48 (3): 752-9. -
Hruby, S. et al. (1987) Monoclonal antibodies reactive with myelin basic protein.
Mol Immunol. 24 (12): 1359-64. -
Relvas, J.B. et al. (2001) Expression of dominant-negative and chimeric subunits reveals an essential role for beta1 integrin during myelination.
Curr Biol. 11: 1039-43. -
Massa, P.T. et al. (2002) Critical role for protein tyrosine phosphatase SHP-1 in controlling infection of central nervous system glia and demyelination by Theiler's murine encephalomyelitis virus.
J Virol. 76:8335-46. -
Massa, P.T. et al. (2004) Dysmyelination and reduced myelin basic protein gene expression by oligodendrocytes of SHP-1-deficient mice.
J Neurosci Res. 77: 15-25. -
Homchaudhuri L et al. (2009) Influence of membrane surface charge and post-translational modifications to myelin basic protein on its ability to tether the Fyn-SH3 domain to a membrane in vitro.
Biochemistry. 48 (11): 2385-93. -
Relucio, J. et al. (2009) Laminin alters fyn regulatory mechanisms and promotes oligodendrocyte development.
J Neurosci. 29: 11794-806.
View The Latest Product References
-
Savvaki, M. et al. (2010) The expression of TAG-1 in glial cells is sufficient for the formation of the juxtaparanodal complex and the phenotypic rescue of tag-1 homozygous mutants in the CNS.
J Neurosci. 30: 13943-54. -
Pohl, H.B. et al. (2011) Genetically induced adult oligodendrocyte cell death is associated with poor myelin clearance, reduced remyelination, and axonal damage.
J Neurosci. 31 (3): 1069-80. -
Laursen, L.S. et al. (2011) Translation of myelin basic protein mRNA in oligodendrocytes is regulated by integrin activation and hnRNP-K.
J Cell Biol. 192: 797-811. -
Monk, K.R. et al. (2011) Gpr126 is essential for peripheral nerve development and myelination in mammals.
Development. 138: 2673-80. -
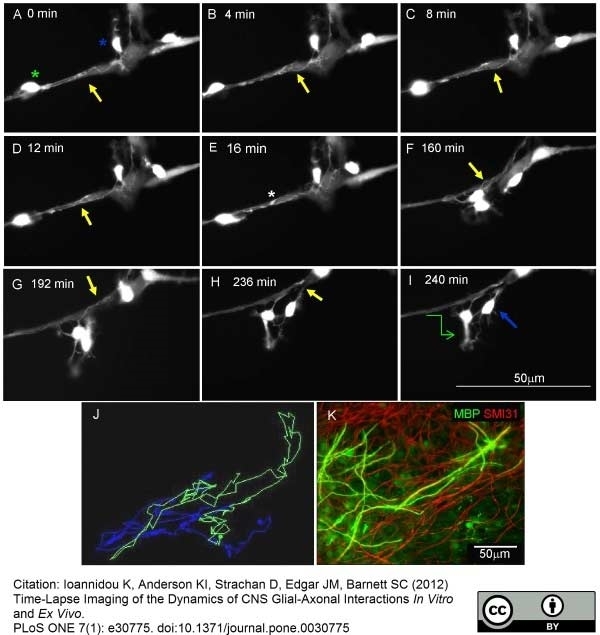
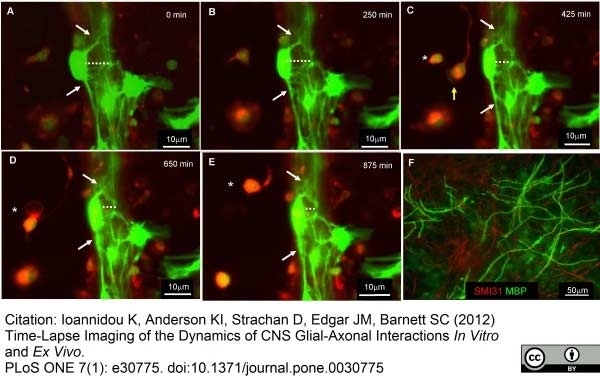
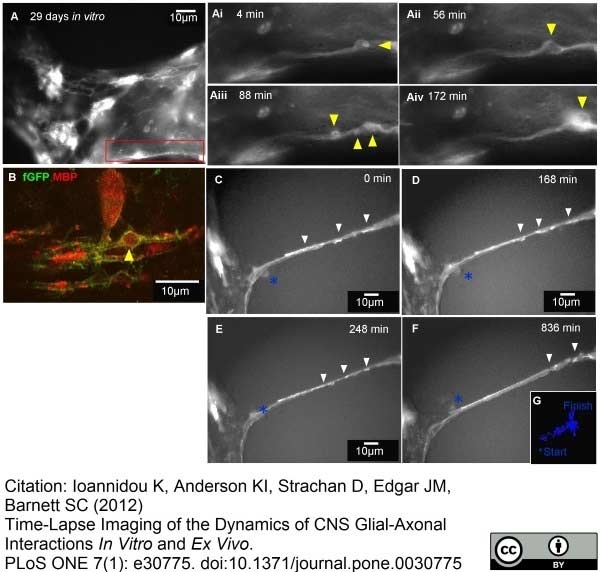
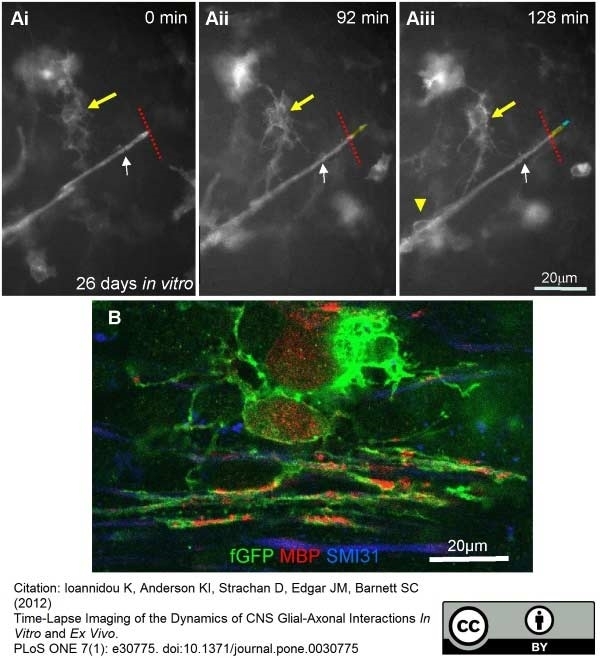
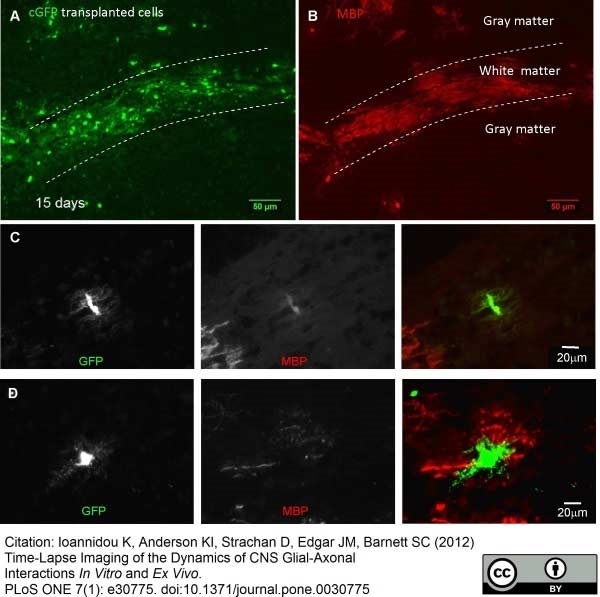
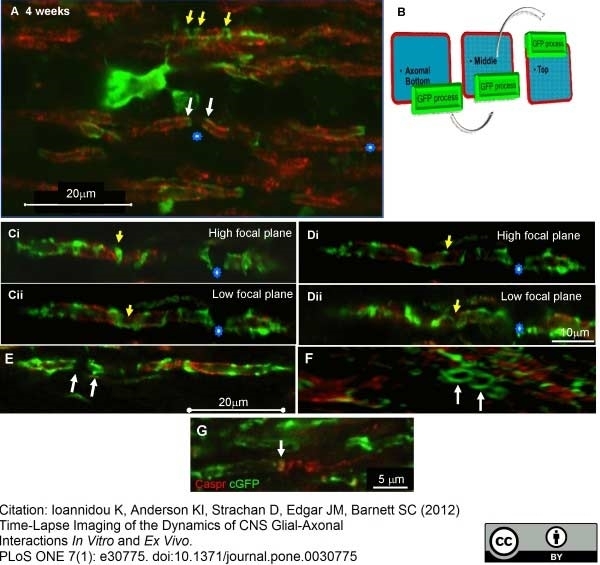
Ioannidou, K. et al. (2012) Time-lapse imaging of the dynamics of CNS glial-axonal interactions in vitro and ex vivo.
PLoS One. 7: e30775. -
Horn, M. et al. (2012) Myelin is dependent on the Charcot-Marie-Tooth Type 4H disease culprit protein FRABIN/FGD4 in Schwann cells.
Brain. 135 (Pt 12): 3567-83. -
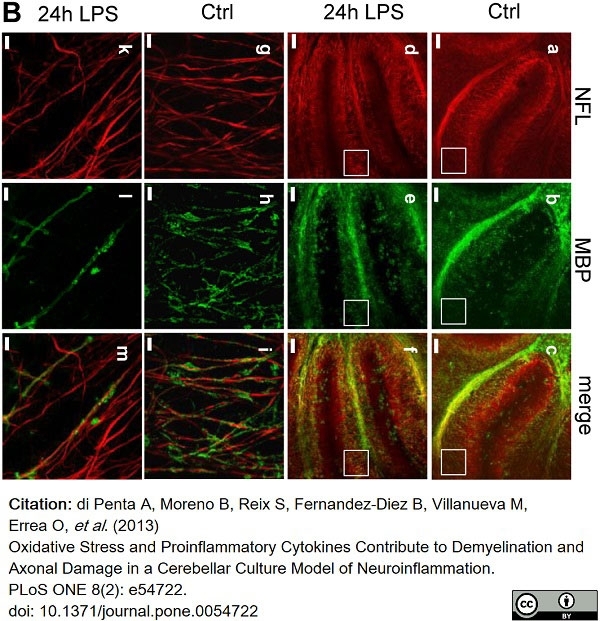
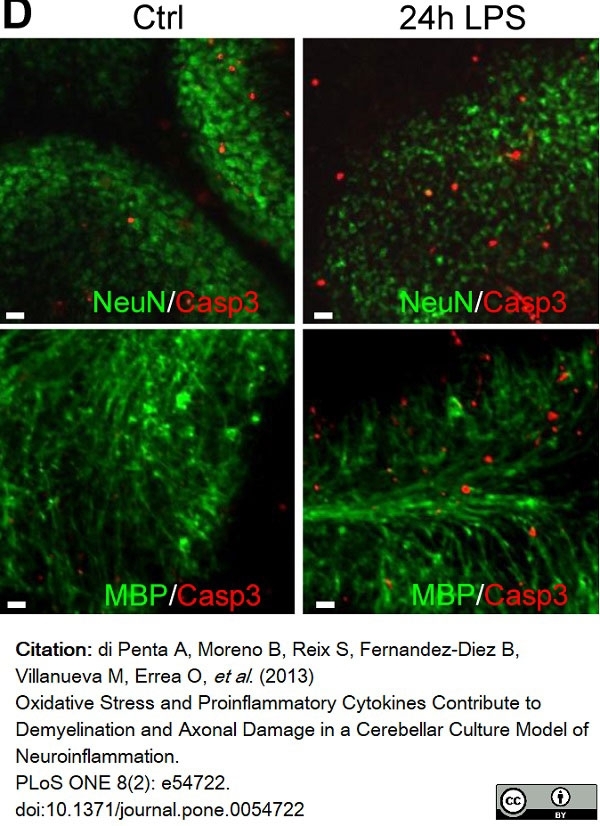
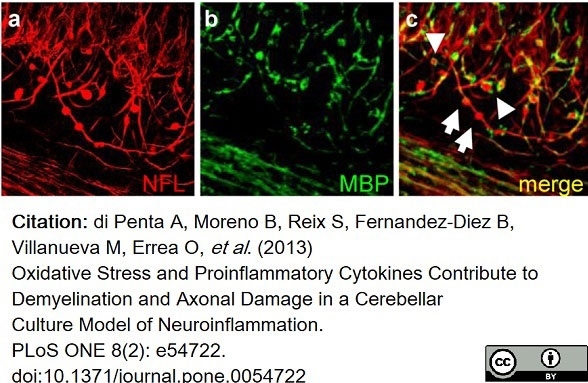
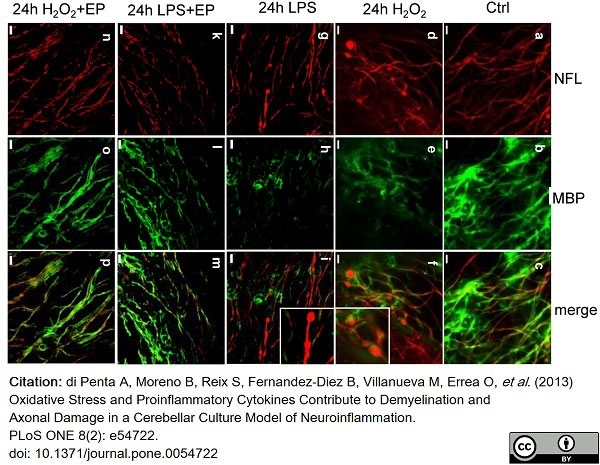
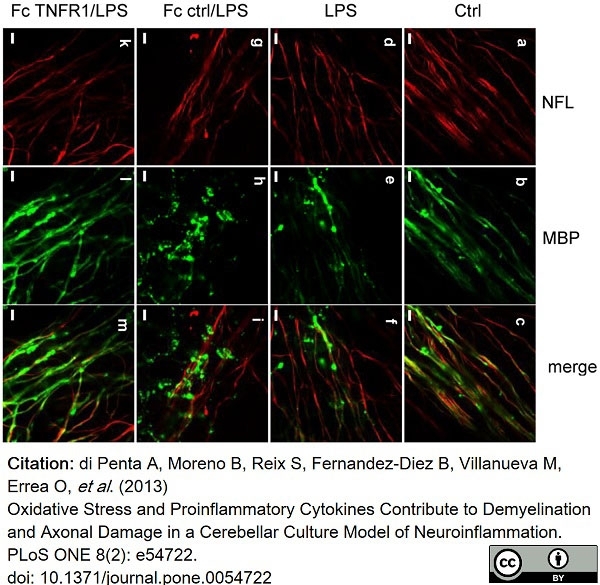
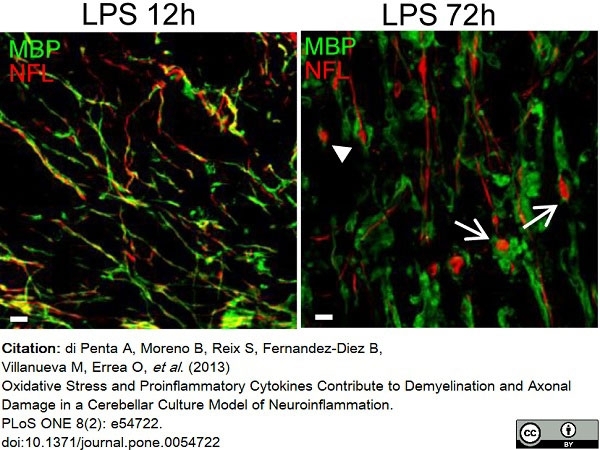
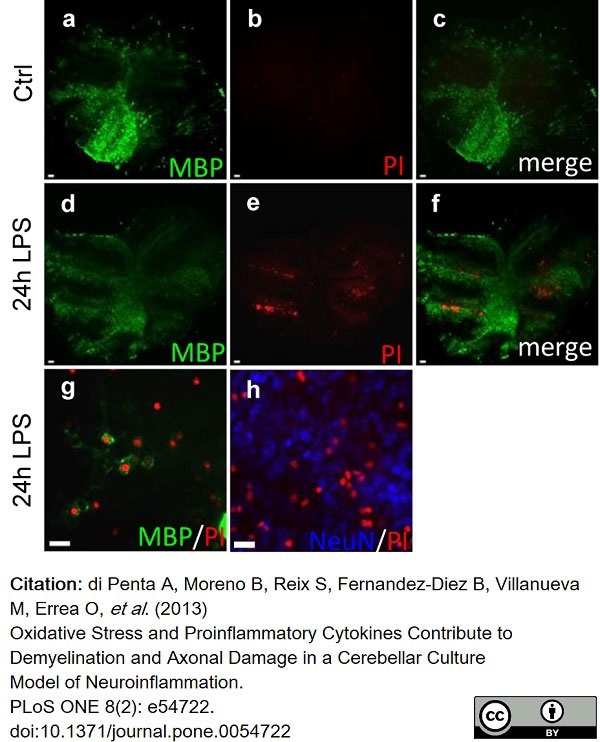
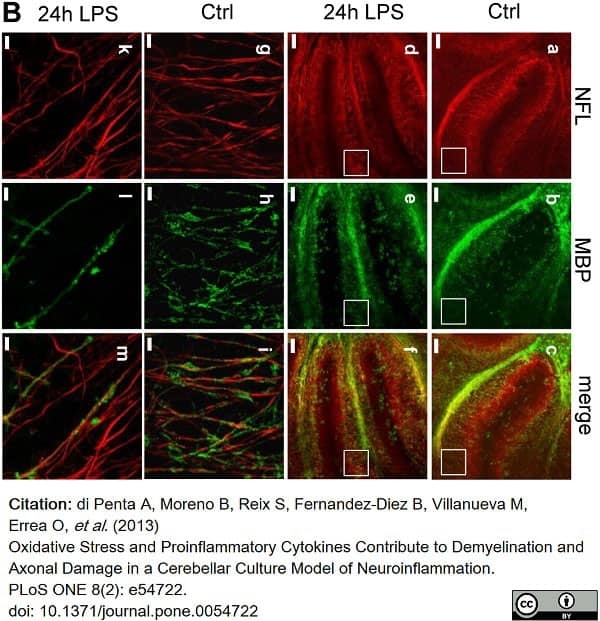
di Petna, A. et al. (2013) Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation.
PLoS One. 8 (2): e54722. -
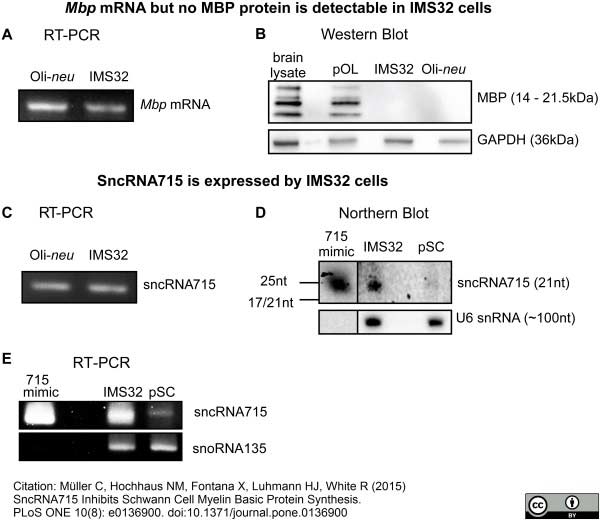
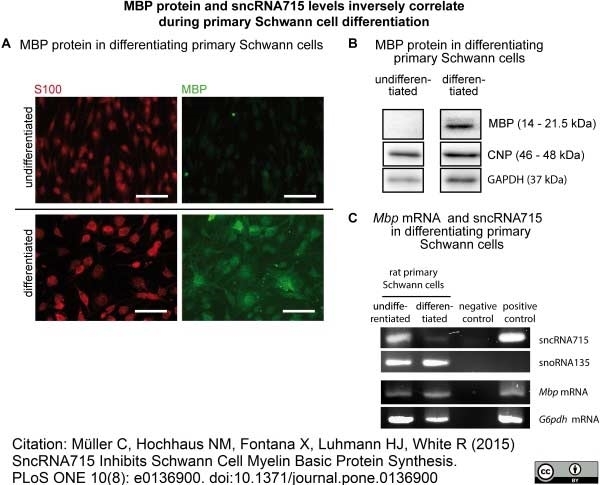
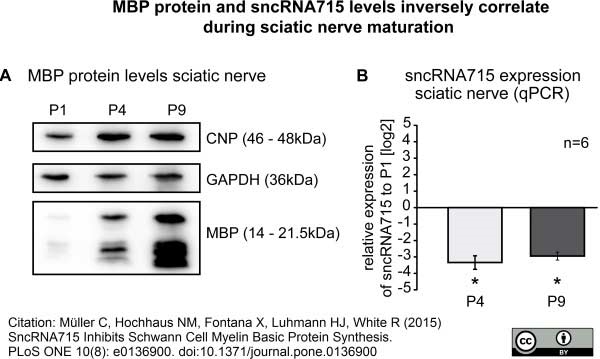
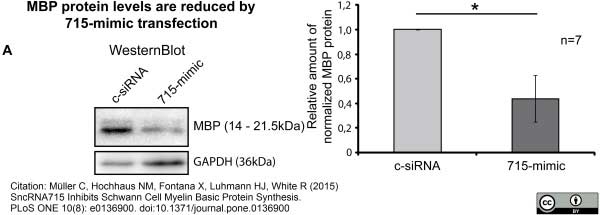
Müller, C. et al. (2015) SncRNA715 Inhibits Schwann Cell Myelin Basic Protein Synthesis.
PLoS One. 10 (8): e0136900. -
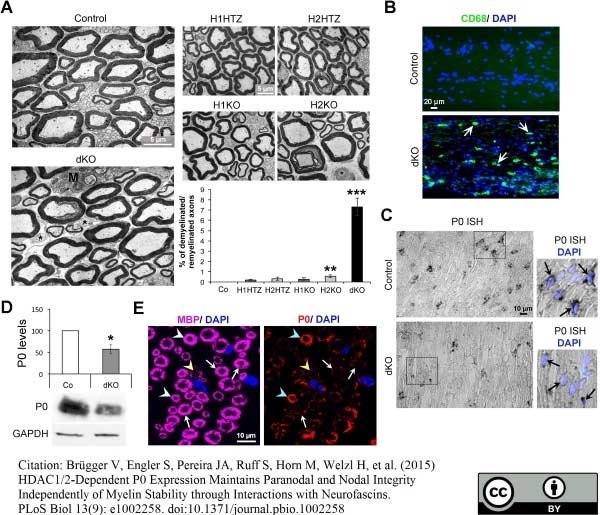
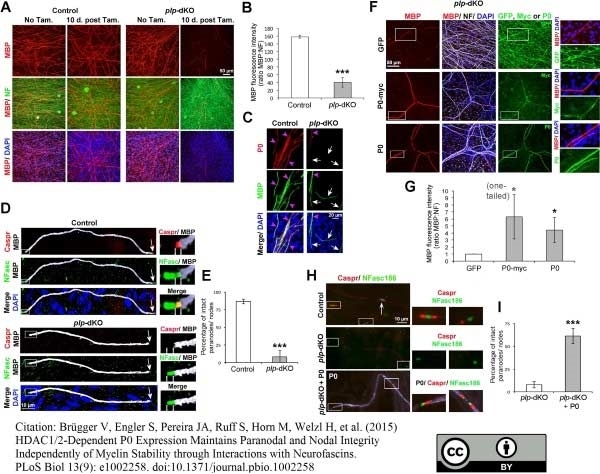
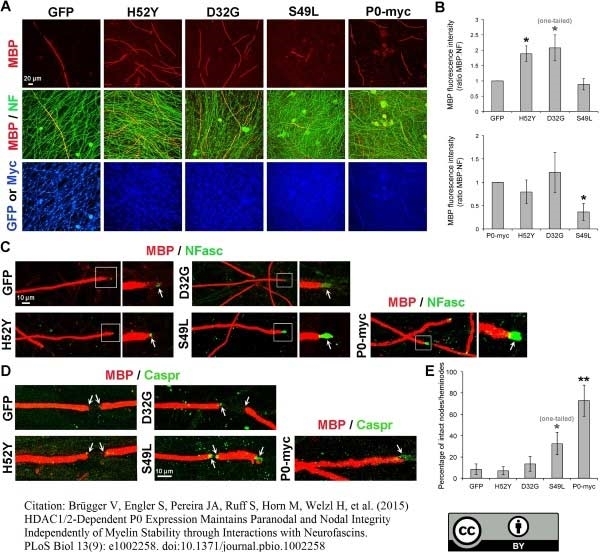
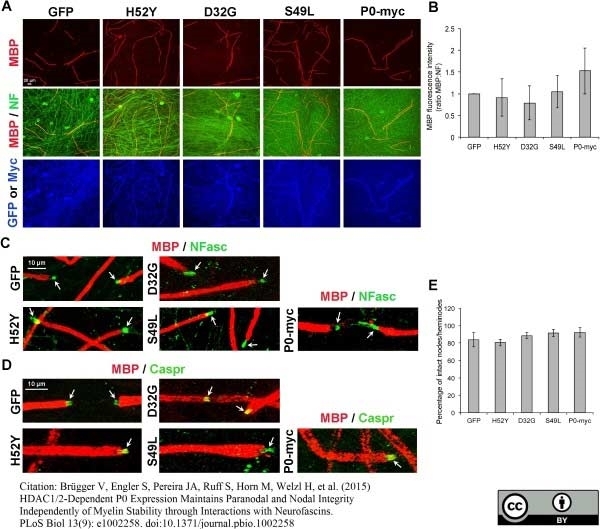
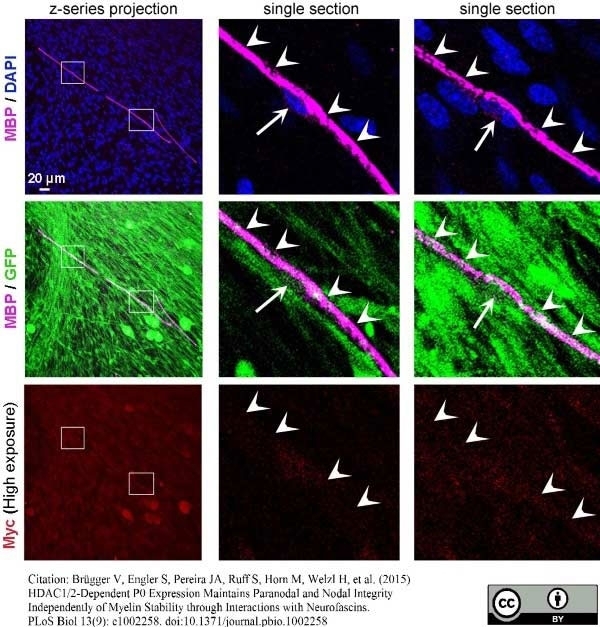
Brügger V et al. (2015) HDAC1/2-Dependent P0 Expression Maintains Paranodal and Nodal Integrity Independently of Myelin Stability through Interactions with Neurofascins.
PLoS Biol. 13 (9): e1002258. -
Natrajan, M.S. et al. (2015) Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination.
Brain. 138(Pt 12):3581-97 -
Rittchen S et al. (2015) Myelin repair in vivo is increased by targeting oligodendrocyte precursor cells with nanoparticles encapsulating leukaemia inhibitory factor (LIF).
Biomaterials. 56: 78-85. -
Friess, M. et al. (2016) Intracellular ion signaling influences myelin basic protein synthesis in oligodendrocyte precursor cells.
Cell Calcium. 60 (5): 322-30. -
Fernandes, A.R. & Chari, D.M. (2016) Part II: Functional delivery of a neurotherapeutic gene to neural stem cells using minicircle DNA and nanoparticles: Translational advantages for regenerative neurology.
J Control Release. 238: 300-10. -
Crawford, A.H. et al. (2016) Pre-Existing Mature Oligodendrocytes Do Not Contribute to Remyelination following Toxin-Induced Spinal Cord Demyelination.
Am J Pathol. 186 (3): 511-6. -
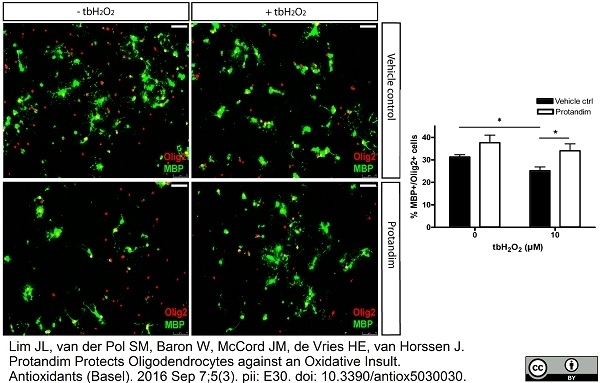
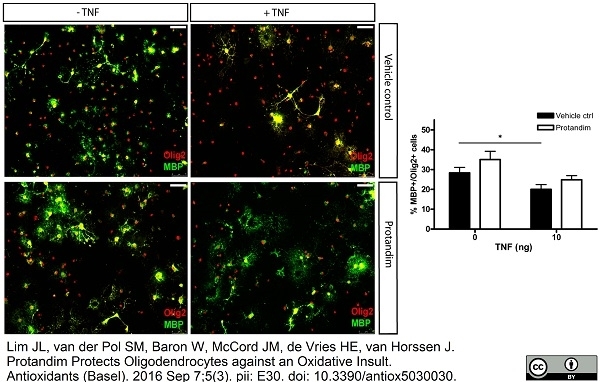
Lim, J.L. et al. (2016) Protandim Protects Oligodendrocytes against an Oxidative Insult.
Antioxidants (Basel). 5 (3): pii: E30. -
Isoda, M. et al. (2016) Robust production of human neural cells by establishing neuroepithelial-like stem cells from peripheral blood mononuclear cell-derived feeder-free iPSCs under xeno-free conditions.
Neurosci Res. 110: 18-28. -
Fernandes, A.R. & Chari, D.M. (2016) Part I: Minicircle vector technology limits DNA size restrictions on ex vivo gene delivery using nanoparticle vectors: Overcoming a translational barrier in neural stem cell therapy.
J Control Release. 238: 289-99. -
Moreno, B. et al. (2017) Methylthioadenosine promotes remyelination by inducing oligodendrocyte differentiation
Mult Scler Demyel Disord. 2, 3. -
Qin, J. et al. (2017) GD1a Overcomes Inhibition of Myelination by Fibronectin via Activation of Protein Kinase A: Implications for Multiple Sclerosis.
J Neurosci. 37 (41): 9925-38. -
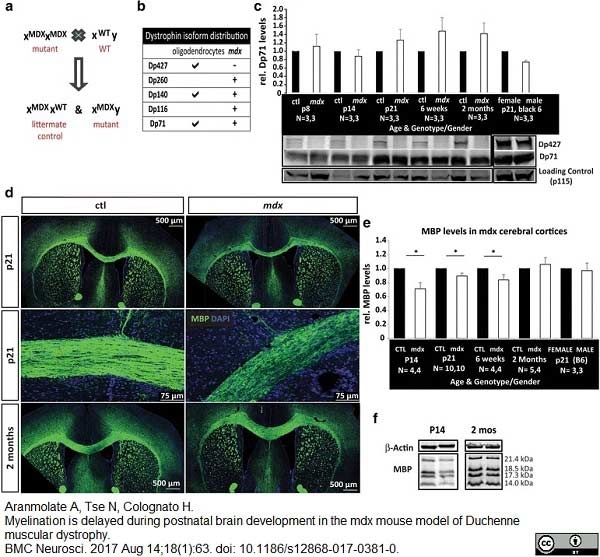
Aranmolate, A. et al. (2017) Myelination is delayed during postnatal brain development in the mdx mouse model of Duchenne muscular dystrophy.
BMC Neurosci. 18 (1): 63. -
Yu, Q. et al. (2017) Strain differences in cuprizone induced demyelination.
Cell Biosci. 7: 59. -
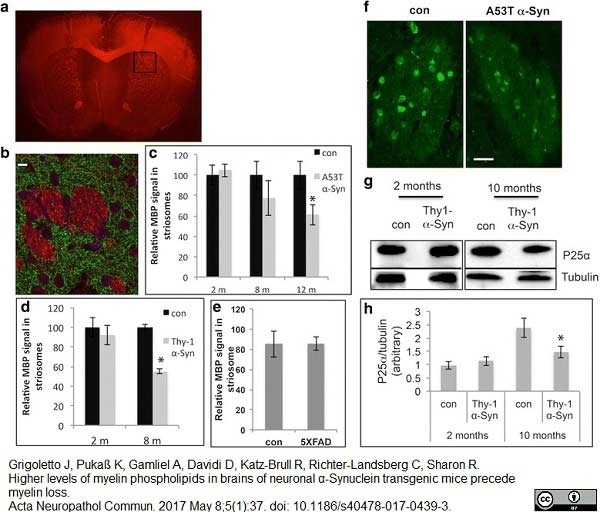
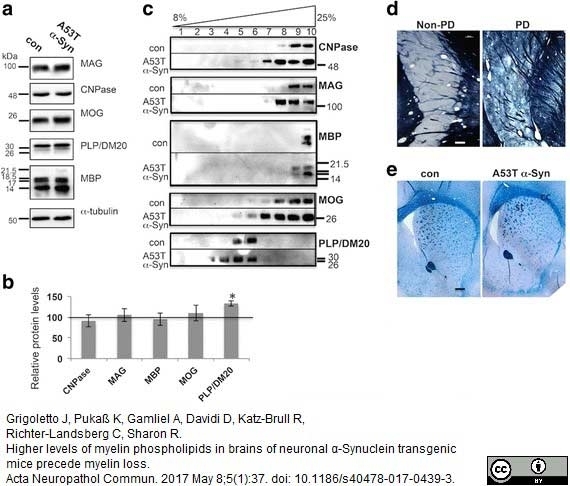
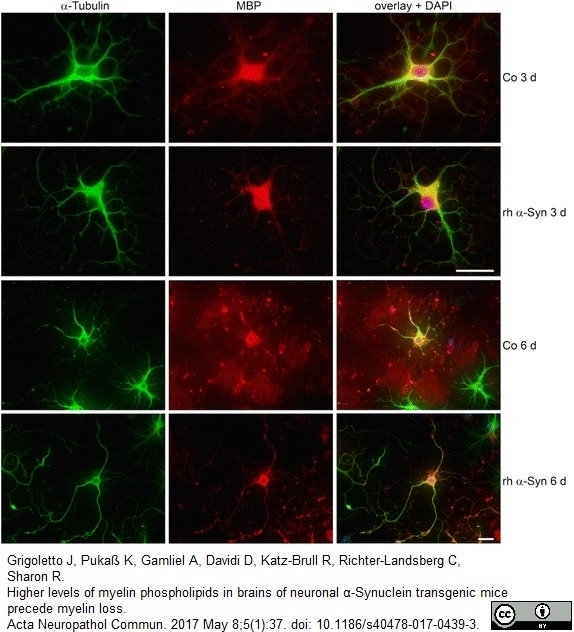
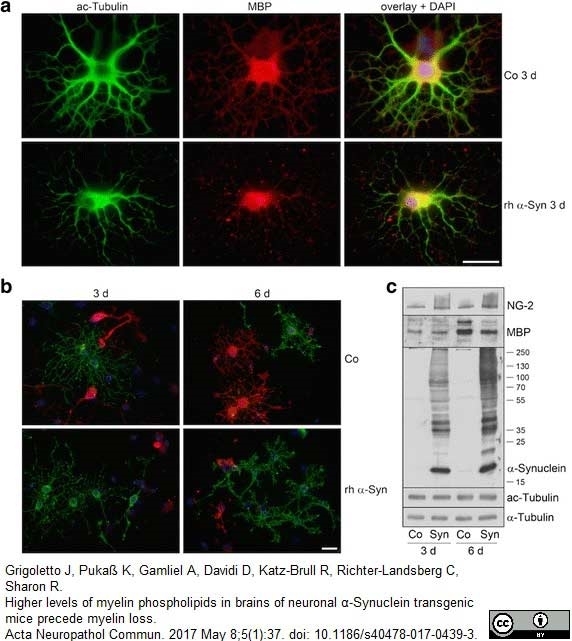
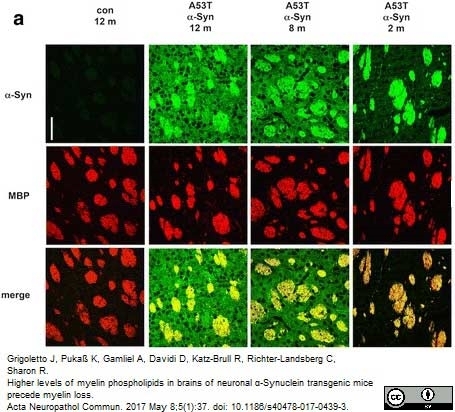
Grigoletto, J. et al. (2017) Higher levels of myelin phospholipids in brains of neuronal α-Synuclein transgenic mice precede myelin loss.
Acta Neuropathol Commun. 5 (1): 37. -
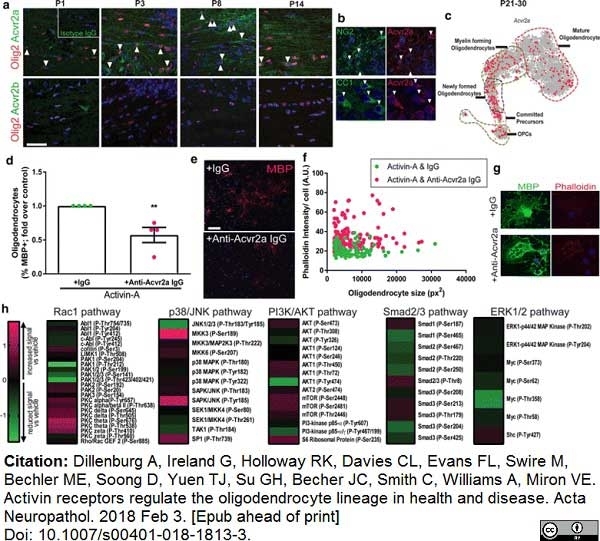
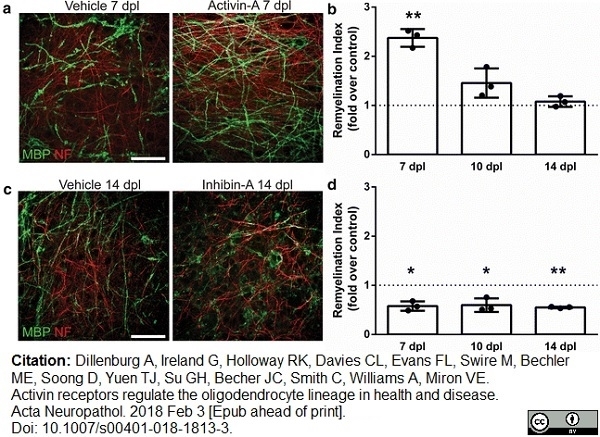
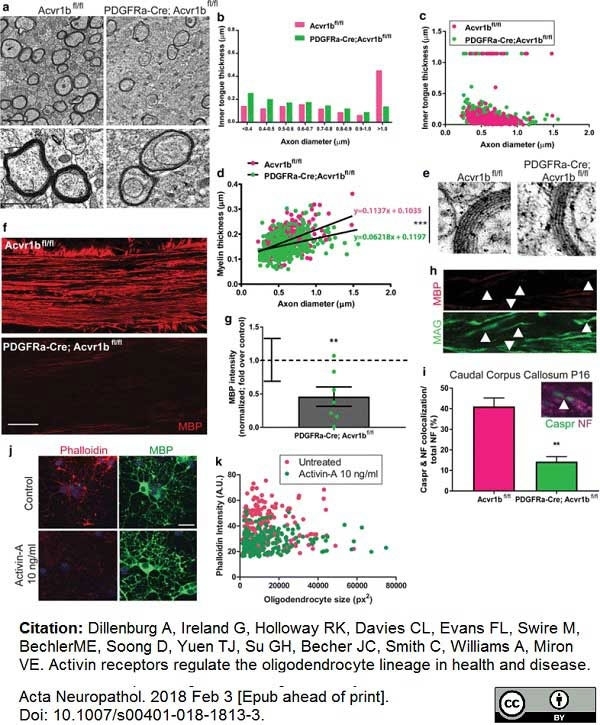
Dillenburg, A. et al. (2018) Activin receptors regulate the oligodendrocyte lineage in health and disease.
Acta Neuropathol. 135 (6): 887-906. -
Sekizar, S. & Williams, A. (2019) Ex Vivo Slice Cultures to Study Myelination, Demyelination, and Remyelination in Mouse Brain and Spinal Cord.
Methods Mol Biol. 1936: 169-83. -
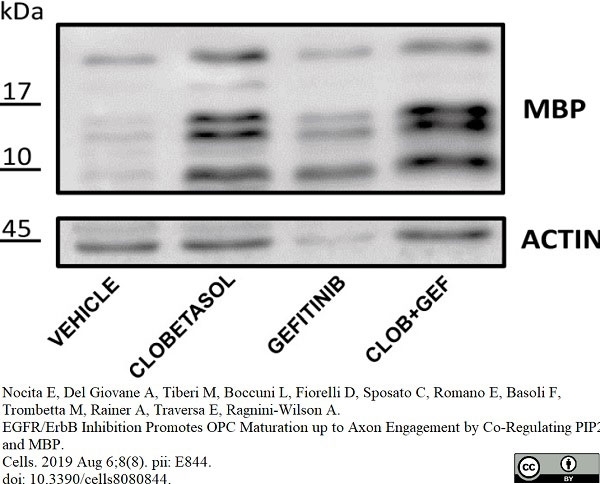
Nocita, E. et al. (2019) EGFR/ErbB Inhibition Promotes OPC Maturation up to Axon Engagement by Co-Regulating PIP2 and MBP.
Cells. 8 (8): 844. -
Martinez-rachadell, L. et al. (2019) Cell-specific expression of insulin/insulin-like growth factor-I receptor hybrids in the mouse brain.
Growth Horm IGF Res. 45: 25-30. -
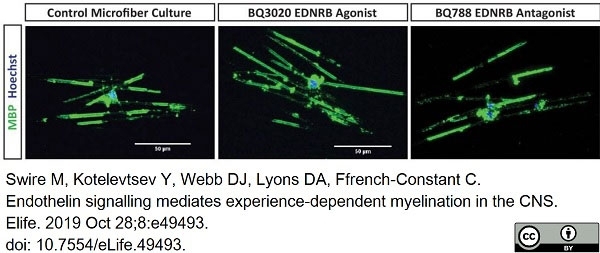
Swire, M. et al. (2019) Endothelin signalling mediates experience-dependent myelination in the CNS.
Elife. 8:e49493 -
Vogel, J.K. et al. (2020) Sox9 overexpression exerts multiple stage-dependent effects on mouse spinal cord development.
Glia. 68 (5): 932-46. -
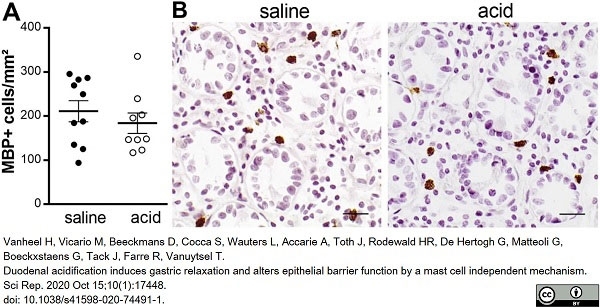
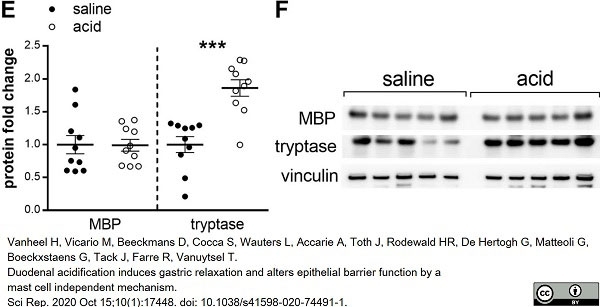
Vanheel, H. et al. (2020) Duodenal acidification induces gastric relaxation and alters epithelial barrier function by a mast cell independent mechanism.
Sci Rep. 10 (1): 17448. -
Kerman, B.E. et al. (2020) Motoneuron expression profiling identifies an association between an axonal splice variant of HDGF-related protein 3 and peripheral myelination.
J Biol Chem. 295 (34): 12233-46. -
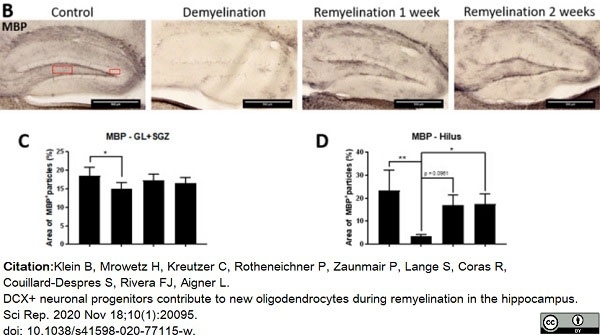
Klein, B. et al. (2020) DCX+ neuronal progenitors contribute to new oligodendrocytes during remyelination in the hippocampus.
Sci Rep. 10 (1): 20095. -
Yetiş, Ç. et al. (2020) Myelin detection in fluorescence microscopy images using machine learning.
J Neurosci Methods. 346: 108946. -
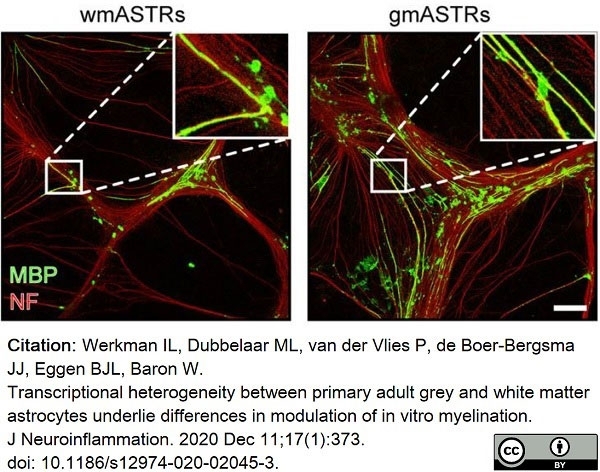
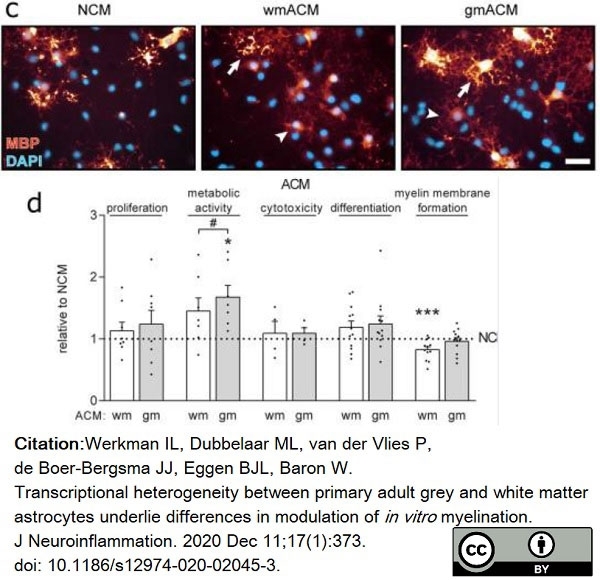
Werkman, I.L. et al. (2020) Transcriptional heterogeneity between primary adult grey and white matter astrocytes underlie differences in modulation of in vitro myelination.
J Neuroinflammation. 17 (1): 373. -
Melero-Jerez, C. et al. (2021) Myeloid-derived suppressor cells support remyelination in a murine model of multiple sclerosis by promoting oligodendrocyte precursor cell survival, proliferation, and differentiation.
Glia. 69 (4): 905-24. -
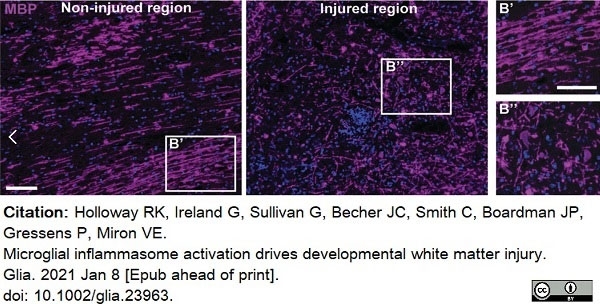
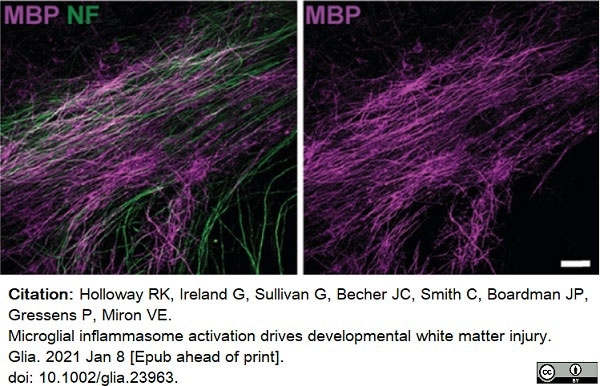
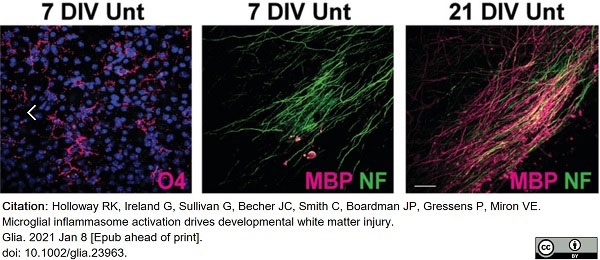
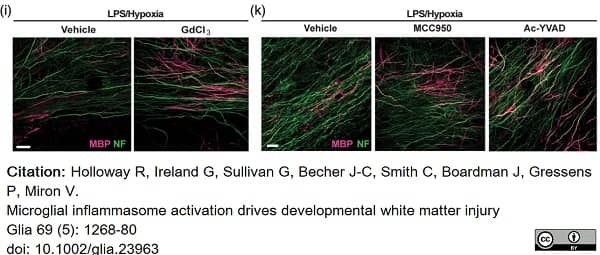
Holloway, R.K. et al. (2021) Microglial inflammasome activation drives developmental white matter injury.
Glia. 69 (5): 1268-80. -
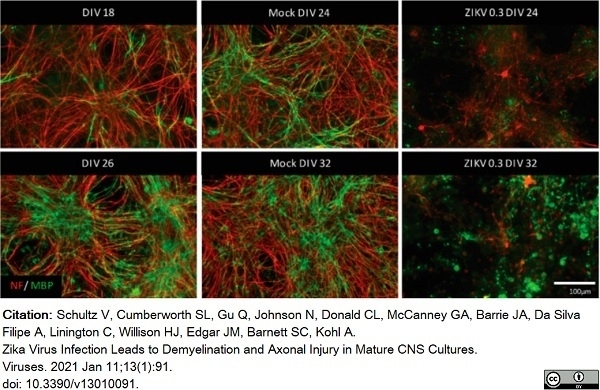
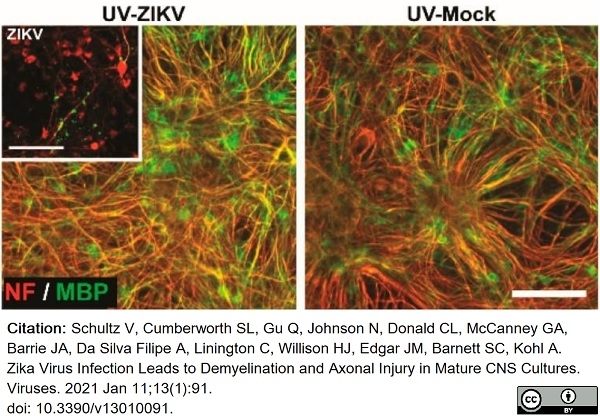
Schultz, V. et al. (2021) Zika Virus Infection Leads to Demyelination and Axonal Injury in Mature CNS Cultures.
Viruses. 13 (1): 91. -
Meireles, A.M. et al. (2018) The Lysosomal Transcription Factor TFEB Represses Myelination Downstream of the Rag-Ragulator Complex.
Dev Cell. 47 (3): 319-330.e5. -
Lloyd, A.F. et al. (2019) Central nervous system regeneration is driven by microglia necroptosis and repopulation.
Nat Neurosci. 22 (7): 1046-52. -
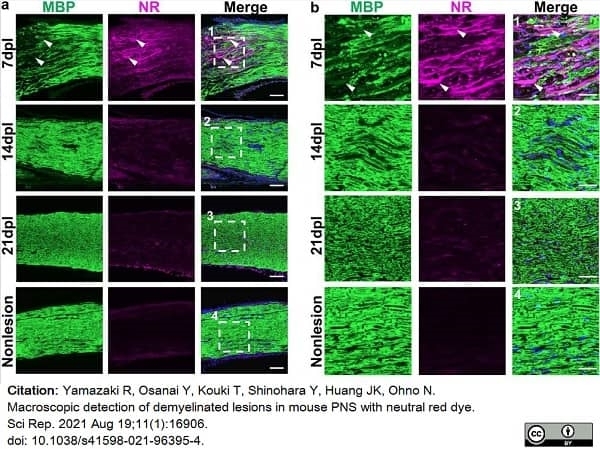
Yamazaki, R. et al. (2021) Macroscopic detection of demyelinated lesions in mouse PNS with neutral red dye.
Sci Rep. 11 (1): 16906. -
Alhajlah, S. et al. (2021) Overexpression of Reticulon 3 Enhances CNS Axon Regeneration and Functional Recovery after Traumatic Injury.
Cells. 10 (8): 2015. -
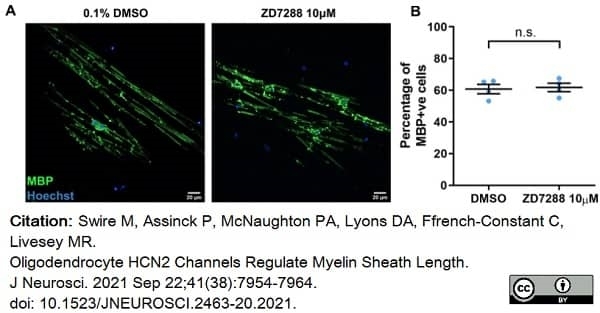
Swire, M. et al. (2021) Oligodendrocyte HCN2 Channels Regulate Myelin Sheath Length.
J Neurosci. 41 (38): 7954-64. -
Niu, J. et al. (2021) Oligodendroglial ring finger protein Rnf43 is an essential injury-specific regulator of oligodendrocyte maturation.
Neuron. 109 (19): 3104-18.e6. -
Moyon, S. et al. (2021) TET1-mediated DNA hydroxymethylation regulates adult remyelination in mice.
Nat Commun. 12 (1): 3359. -
Bechler, M.E. (2019) A Neuron-Free Microfiber Assay to Assess Myelin Sheath Formation.
Methods Mol Biol. 1936: 97-110. -
Sekizar, S. & Williams, A. (2019) Ex Vivo. Slice Cultures to Study Myelination, Demyelination, and Remyelination in Mouse Brain and Spinal Cord.
Methods Mol Biol. 1936: 169-83. -
Li, S. et al. (2019) Induction of immunological tolerance to myelinogenic glial-restricted progenitor allografts.
Brain. 142 (11): 3456-72. -
Berghoff, S.A. et al. (2021) Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis.
Nat Neurosci. 24 (1): 47-60. -
Kalafatakis, I. et al. (2021) The beneficial role of the synthetic microneurotrophin BNN20 in a focal demyelination model.
J Neurosci Res. 99 (5): 1474-95. -
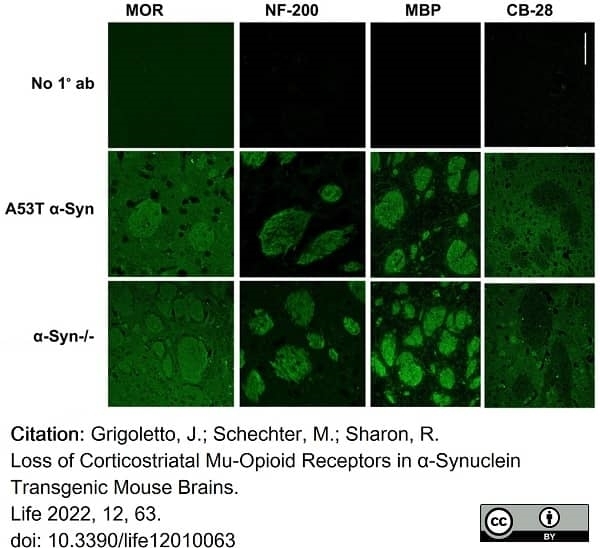
Grigoletto, J. et al. (2022) Loss of Corticostriatal Mu-Opioid Receptors in α-Synuclein Transgenic Mouse Brains.
Life (Basel). 12 (1): 63. -
Poggi, G. et al. (2022) Effects of chronic social stress on oligodendrocyte proliferation-maturation and myelin status in prefrontal cortex and amygdala in adult mice
Neurobiol Stress. 18: 100451. -
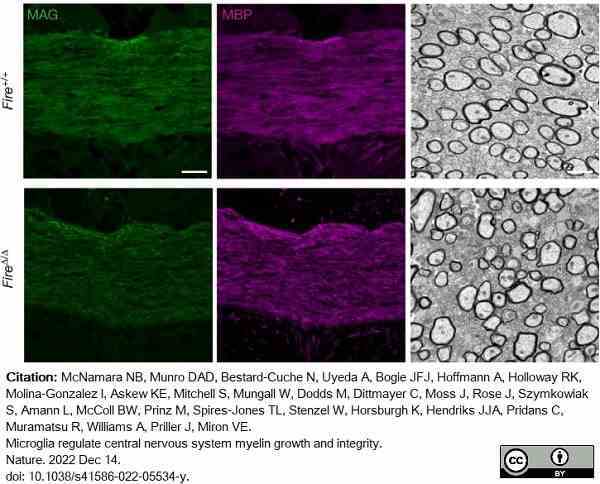
McNamara, N.B. et al. (2023) Microglia regulate central nervous system myelin growth and integrity.
Nature. 613 (7942): 120-9. -
Aber, E.R. et al. (2022) Oligodendroglial macroautophagy is essential for myelin sheath turnover to prevent neurodegeneration and death.
Cell Rep. 41 (3): 111480. -
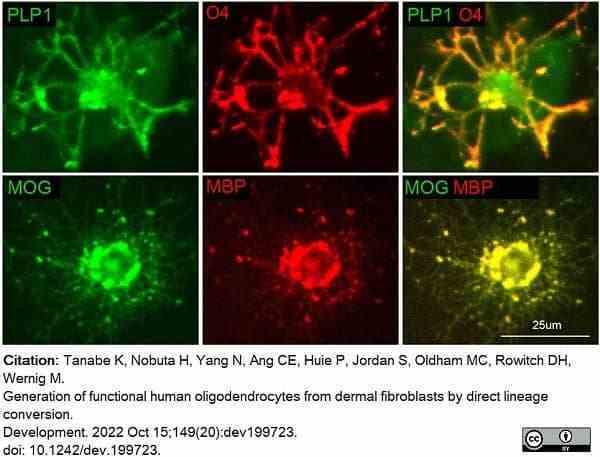
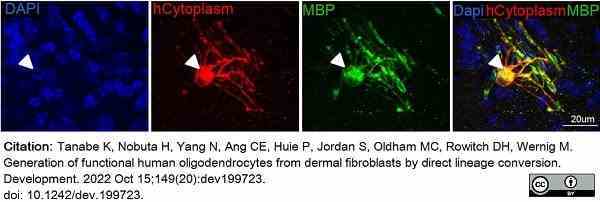
Tanabe, K. et al. (2022) Generation of functional human oligodendrocytes from dermal fibroblasts by direct lineage conversion.
Development. 149 (20): dev199723. -
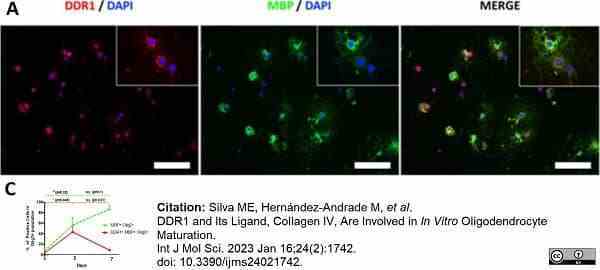
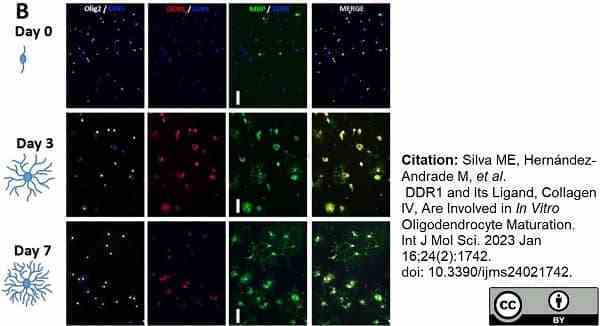
Silva, M.E. et al. (2023) DDR1 and Its Ligand, Collagen IV, Are Involved in In Vitro Oligodendrocyte Maturation.
Int J Mol Sci. 24 (2): 1742. -
Freire, M.A.M. et al. (2023) Astrocytosis, Inflammation, Axonal Damage and Myelin Impairment in the Internal Capsule following Striatal Ischemic Injury
Cells. 12 (3): 457. -
Holloway, R.K. et al. (2023) Localized microglia dysregulation impairs central nervous system myelination in development.
Acta Neuropathol Commun. 11 (1): 49. -
Rocha, D.N. et al. (2023) It takes two to remyelinate: A bioengineered platform to study astrocyte-oligodendrocyte crosstalk and potential therapeutic targets in remyelination.
Biomater Adv. 151: 213429. -
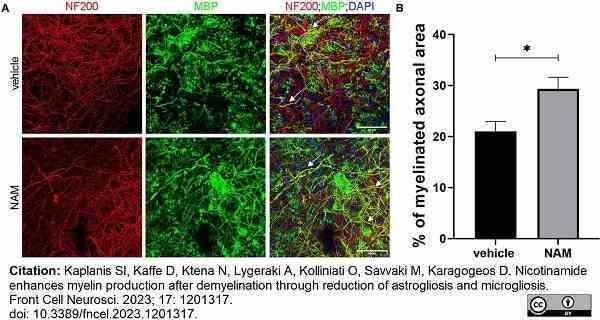
Kaplanis, S.I. et al. (2023) Nicotinamide enhances myelin production after demyelination through reduction of astrogliosis and microgliosis.
Front Cell Neurosci. 17: 1201317. -
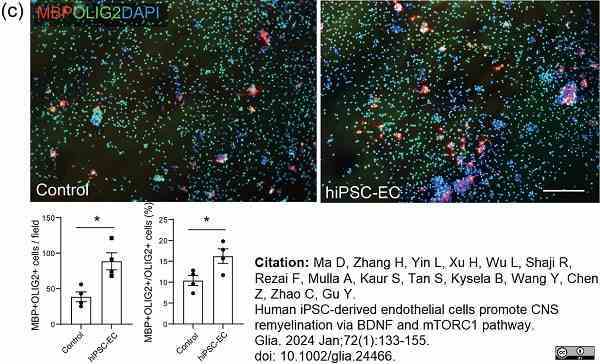
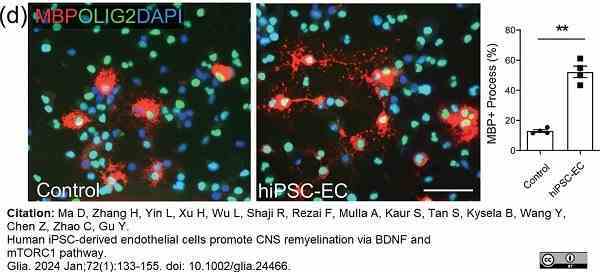
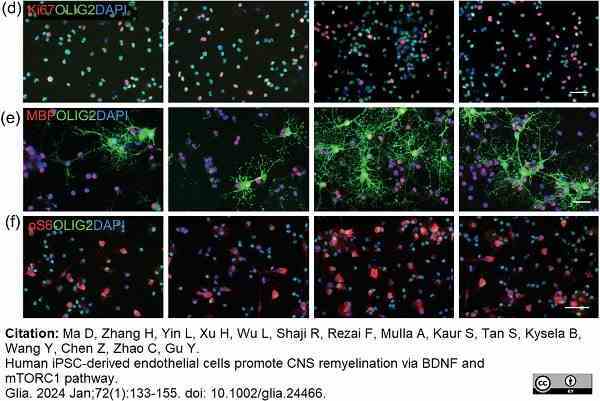
Ma, D. et al. (2023) Human iPSC-derived endothelial cells promote CNS remyelination via BDNF and mTORC1 pathway
Glia. 72(1):133-55. -
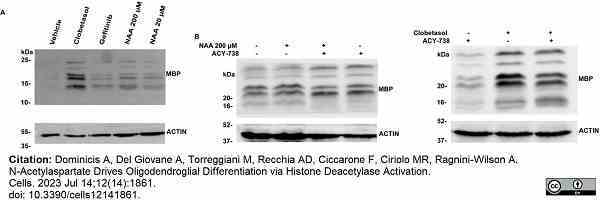
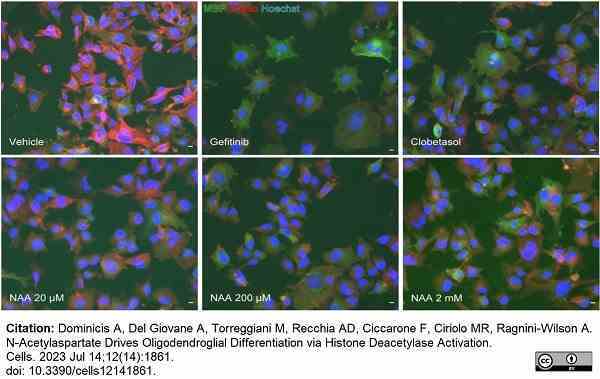
Dominicis, A. et al. (2023) N-Acetylaspartate Drives Oligodendroglial Differentiation via Histone Deacetylase Activation.
Cells. 12 (14): 1861. -
Molina-Gonzalez, I. et al. (2023) Astrocyte-oligodendrocyte interaction regulates central nervous system regeneration.
Nat Commun. 14 (1): 3372. -
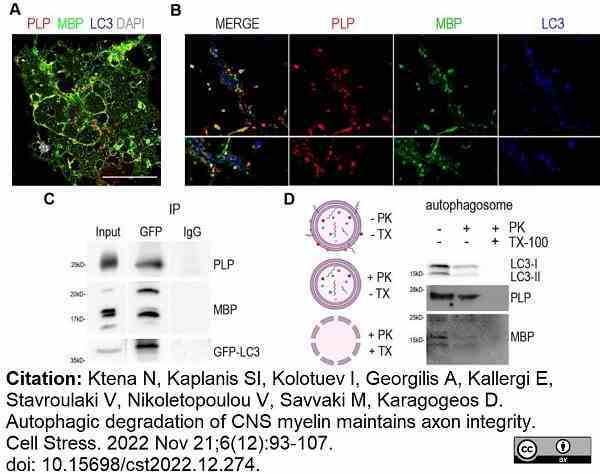
Ktena, N. et al. (2022) Autophagic degradation of CNS myelin maintains axon integrity.
Cell Stress. 6 (12): 93-107. -
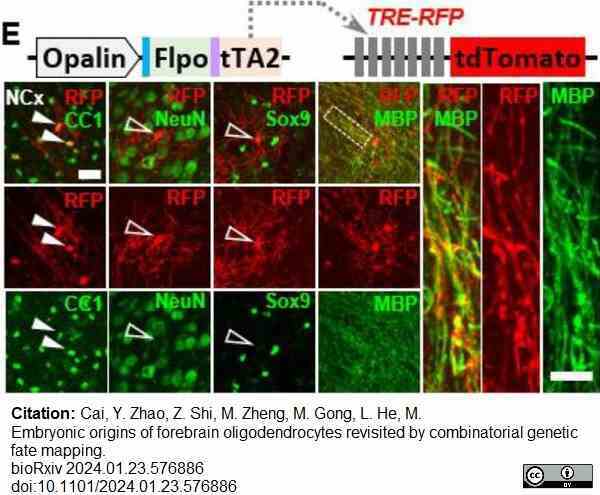
Cai, Y. et al. (2024) Embryonic origins of forebrain oligodendrocytes revisited by combinatorial genetic fate mapping.
bioRχiv. 23 Jan [Epub ahead of print]. -
Seo, T. et al. (2023) MARCH family E3 ubiquitin ligases selectively target and degrade cadherin family proteins
bioRχiv 10 Aug [Epub ahead of print]. -
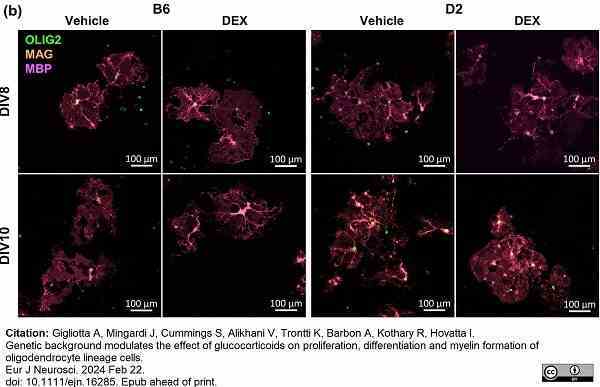
Gigliotta, A. et al. (2024) Genetic background modulates the effect of glucocorticoids on proliferation, differentiation and myelin formation of oligodendrocyte lineage cells.
Eur J Neurosci. Feb 22 [Epub ahead of print]. -
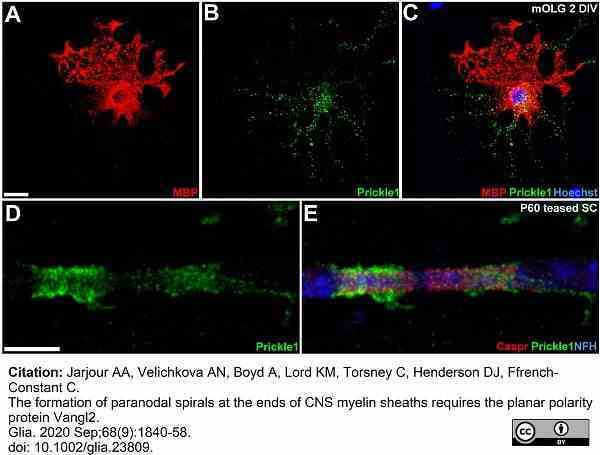
Jarjour, A.A. et al. (2020) The formation of paranodal spirals at the ends of CNS myelin sheaths requires the planar polarity protein Vangl2.
Glia. 68 (9): 1840-1858. -
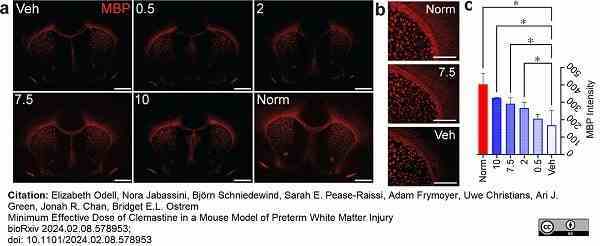
Odell, E. et al. (2024) Minimum Effective Dose of Clemastine in a Mouse Model of Preterm White Matter Injury.
bioRχiv. Mar 01 [Epub ahead of print].
- Synonyms
- Myelin Basic Protein
- RRID
- AB_325004
- UniProt
- P02687
- Entrez Gene
- MBP
- GO Terms
- GO:0005886 plasma membrane
- GO:0005515 protein binding
- GO:0019911 structural constituent of myelin sheath
- GO:0043209 myelin sheath
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Bovine ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up