Affinity and Avidity of Antibodies

- On This Page
- Antibody affinity
- Antibody avidity
- Further useful reading
Antigen-antibody interactions are non-covalent and reversible, formed by a combination of hydrogen bonds, hydrophobic interactions, electrostatic and van der Waals forces.
When describing the strength of the antigen-antibody complex, affinity and avidity are always mentioned.
Antibody Affinity
Affinity measures the strength of interaction between an epitope and an antibody’s antigen binding site. It is defined by the same basic thermodynamic principles that govern any reversible biomolecular interaction:
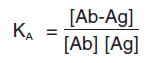
- KA = affinity constant
- [Ab] = molar concentration of unoccupied binding sites on the antibody
- [Ag] = molar concentration of unoccupied binding sites on the antigen
- [Ab-Ag] = molar concentration of the antibody-antigen complex
In other words, KA describes how much antibody-antigen complex exists at the point when equilibrium is reached. The time taken for this to occur depends on rate of diffusion and is similar for every antibody. However, high-affinity antibodies will bind a greater amount of antigen in a shorter period of time than low-affinity antibodies. KA can therefore vary widely for antibodies from below 105 mol-1 to above 1012 mol-1, and can be influenced by factors including pH, temperature and buffer composition.
The affinity of monoclonal antibodies can be measured accurately because they are homogeneous and selective for a single epitope. Polyclonal antibodies are heterogeneous and will contain a mixture of antibodies of different affinities recognizing several epitopes – therefore only an average affinity can be determined.
Antibody Avidity
Avidity gives a measure of the overall strength of an antibody-antigen complex. It is dependent on three major parameters:
- Affinity of the antibody for the epitope (see above)
- Valency of both the antibody and antigen
- Structural arrangement of the parts that interact
All antibodies are multivalent e.g. IgGs are bivalent and and IgMs are decavalent. The greater an immunoglobulin’s valency (number of antigen binding sites), the greater the amount of antigen it can bind. Similarly, antigens can demonstrate multivalency because they can bind to more than one antibody. Multimeric interactions between an antibody and an antigen help their stabilization.
A favorable structural arrangement of antibody and antigen can also lead to a more stable antibody-antigen complex as illustrated in Figures 1 and 2.
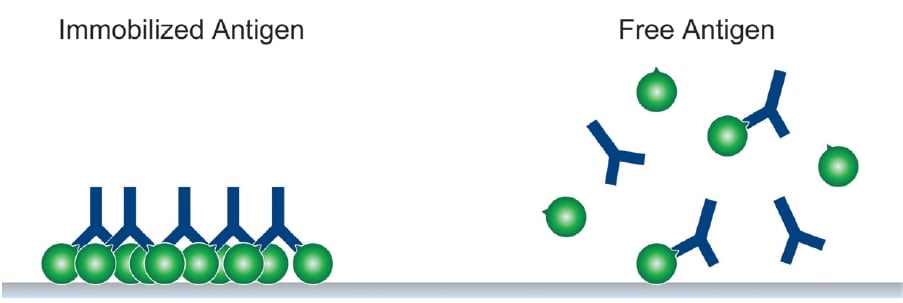
Figure 1. An immobilized antigen (a high local concentration of available epitopes) provides more opportunity for the antibody-antigen complex to form than free antigen in solution over the same time period. Once the first antigen binding arm of an antibody attaches to an antigen on a solid support, the chances of a bivalent interaction are greatly improved. Many immunoassays like Western blotting and ELISA exploit this principle.
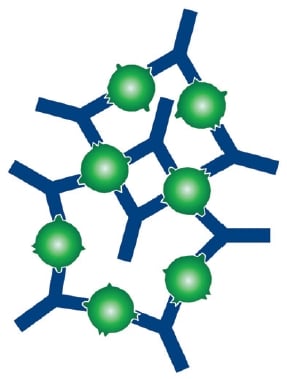
Figure 2. When an antigen is mixed with a polyclonal antibody, multivalent interactions may lead to large, stable (high avidity) structures being formed. This is because the antigen may be bound by several antibodies, each recognizing a different epitope. Polyclonal antibodies are therefore ideal for immunoprecipitation experiments.
Bio-Rad is a global antibody manufacturer and supplier of antibodies, kits and accessories. We provide great customer support for all the products and services we supply so please contact us with any questions on their use or the results you can expect. We can even help source products not currently available or develop them to your specification using our custom antibody services team.