IL-8 antibody | 8M6


Mouse anti Sheep Interleukin-8
- Product Type
- Monoclonal Antibody
- Clone
- 8M6
- Isotype
- IgG2a
- Specificity
- IL-8
| Mouse anti Ovine Interleukin-8 antibody, clone 8M6 recognizes ovine interleukin-8 (IL-8), also known as C-X-C motif chemokine 8. IL-8 is a 79 amino acid ~9-11 kDa chemoattractant for neutrophils, basophils and T-cells. IL-8 is produced by several cell types including neutrophils, monocytes and macrophages in response to inflammatory stimulation. Mouse anti ovine Interleukin-8 antibody, clone 8M6 shows no cross-reactivity with ovine IL-1 beta, IL-6, MCP-1 or TNF alpha. Responses to infectious stimuli may vary among ovine species, the response to Mannheimia haemolytica, a causative agent of pneumonia, peritonitis and gangrenous mastitis in ovids, is exaggerated in Bighorn sheep (Ovis canadensis) compared to domestic sheep (Ovis aries) with significantly elevated IL-8 levels in response to infection (Herndon et al. 2010). Mouse anti ovine interleukin-8 antibody, clone 8M6 has been utilized to identify cells and cell types expressing IL-8 in inflamed porcine tissue (Laursen et al. 2014) showing here also that neutrophils are the predominant cell type expressing IL-8 whilst epithelial and endothelial cells in the vicinity of inflammatory lesions also express the cytokine. Clone 8M6 neutralizes the bioactivity of ovine IL-8. Mouse anti Ovine Interleukin-8 antibody, clone 8M6 has been used in conjunction with Rabbit anti Sheep Interleukin-8 antibody (AHP425) for the development of a sensitive ELISA to measure IL-8 concentrations in bovine samples (Cronin et al. 2015). |
- Target Species
- Sheep
- Species Cross-Reactivity
-
Target Species Cross Reactivity Dog Rabbit Bovine Pig Mustelid Ferret Mink Cat - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Antibody purified from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- <0.1% sodium azide (NaN3)
- Immunogen
- Recombinant ovine IL-8.
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA | 5ug/ml | ||
| Flow Cytometry 1 | 1/10 | ||
| Functional Assays 2 | |||
| Western Blotting |
- 1 Membrane permeabilization is required for this application. The use of Leucoperm (Product Code BUF09) is recommended for this purpose.
- 2 Removal of the preservative is recommended prior to use in functional assays.
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 1x106 cells in 100μl
- ELISA
- Mouse anti Sheep interleukin-8 antibody, clone 8M6 may be used in combination with AHP425 in sandwich ELISA assays for ovine IL-8.
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Goat anti Mouse IgG (H/L):FITC (Multi Species Adsorbed) | STAR117F | F | 0.5 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Goat anti Mouse IgG (H/L):FITC (Multi Species Adsorbed) | ||||||
| Rabbit F(ab')2 anti Mouse IgG:RPE | STAR12A | F | 1 ml |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rabbit F(ab')2 anti Mouse IgG:RPE | ||||||
| Rabbit F(ab')2 anti Mouse IgG:HRP (Human Adsorbed) | STAR13B | C E P RE WB | 1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rabbit F(ab')2 anti Mouse IgG:HRP (Human Adsorbed) | ||||||
| Rabbit F(ab')2 anti Mouse IgG:FITC | STAR9B | F | 1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rabbit F(ab')2 anti Mouse IgG:FITC | ||||||
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG2a Negative Control | MCA929 | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG2a Negative Control | ||||||
References for IL-8 antibody
-
Caswell, J.L. et al. (1998) Expression of the neutrophil chemoattractant interleukin-8 in the lesions of bovine pneumonic pasteurellosis.
Vet Pathol. 35: 124-31. -
Pedersen, L.G. et al. (2002) Identification of monoclonal antibodies that cross-react with cytokines from different animal species.
Vet Immunol Immunopathol. 88 (3-4): 111-22. -
Aasted, B. et al. (2002) Cytokine profiles in peripheral blood mononuclear cells and lymph node cells from piglets infected in utero with porcine reproductive and respiratory syndrome virus.
Clin Diagn Lab Immunol. 9 (6): 1229-34. -
Herndon, C.N. et al. (2010) Differential expression of interleukin-8 by polymorphonuclear leukocytes of two closely related species, Ovis canadensis and Ovis aries, in response to Mannheimia haemolytica infection.
Infect Immun. 78: 3578-84. -
Martel, C.J. & Aasted, B. (2009) Characterization of antibodies against ferret immunoglobulins, cytokines and CD markers.
Vet Immunol Immunopathol. 132:109-15. -
Zelnickova, P. et al. (2008) Age-dependent changes of proinflammatory cytokine production by porcine peripheral blood phagocytes.
Vet Immunol Immunopathol. 124: 367-78. -
Bonnefont, C.M. et al. (2012) Genetic susceptibility to S. aureus mastitis in sheep: differential expression of mammary epithelial cells in response to live bacteria or supernatant.
Physiol Genomics. 44: 403-16. -
Jensen, P.V. et al. (2003) Cytokine profiles in adult mink infected with Aleutian mink disease parvovirus.
J Virol. 77: 7444-51.
View The Latest Product References
-
Singh, B. et al. (2004) Depletion of pulmonary intravascular macrophages inhibits acute lung inflammation.
Am J Physiol Lung Cell Mol Physiol. 286: L363-72. -
Redondo, E. et al. (2013) Induction of Interleukin-8 and Interleukin-12 in Neonatal Ovine Lung Following Experimental Inoculation of Bovine Respiratory Syncytial Virus.
J Comp Pathol. 150: 434-48. -
Laursen, H. et al. (2014) Immunohistochemical detection of interleukin-8 in inflamed porcine tissues.
Vet Immunol Immunopathol. 159: 97-102. -
Boulanger, D. et al. (2003) Increased nuclear factor kappaB activity in milk cells of mastitis-affected cows.
J Dairy Sci. 86: 1259-67. -
Cronin, J.G. et al. (2015) Enzyme linked immunosorbent assay for quantification of bovine interleukin-8 to study infection and immunity in the female genital tract.
Am J Reprod Immunol. 73 (4): 372-82. -
Doull, L. et al. (2015) Late production of CXCL8 in ruminant oro-nasal turbinate cells in response to Chlamydia abortus infection.
Vet Immunol Immunopathol. 168 (1-2): 97-102. -
Rainard, P. et al. (2008) The chemokine CXCL3 is responsible for the constitutive chemotactic activity of bovine milk for neutrophils.
Mol Immunol. 45 (15): 4020-7. -
Stinson LF et al. (2014) Effects of cytokine-suppressive anti-inflammatory drugs on inflammatory activation in ex vivo human and ovine fetal membranes.
Reproduction. 147 (3): 313-20. -
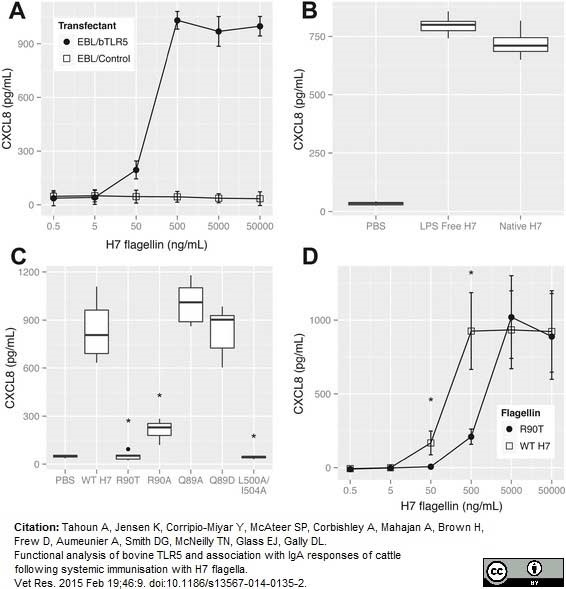
Tahoun, A. et al. (2015) Functional analysis of bovine TLR5 and association with IgA responses of cattle following systemic immunisation with H7 flagella.
Vet Res. 46: 9. -
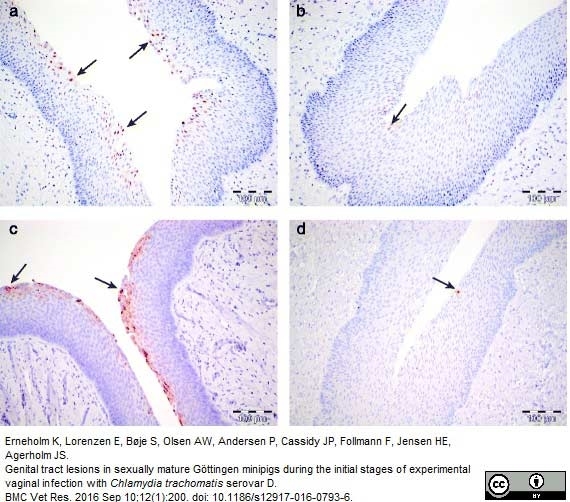
Erneholm, K. et al. (2016) Genital tract lesions in sexually mature Göttingen minipigs during the initial stages of experimental vaginal infection with Chlamydia trachomatis serovar D.
BMC Vet Res. 12 (1): 200. -
Melville, J.M. et al. (2017) Human amnion epithelial cells modulate the inflammatory response to ventilation in preterm lambs.
PLoS One. 12 (3): e0173572. -
Magiri, R. et al. (2019) Innate immune response profiles in pigs injected with vaccine adjuvants polydi(sodium carboxylatoethylphenoxy)phosphazene (PCEP) and Emulsigen.
Vet Immunol Immunopathol. 209: 7-16. -
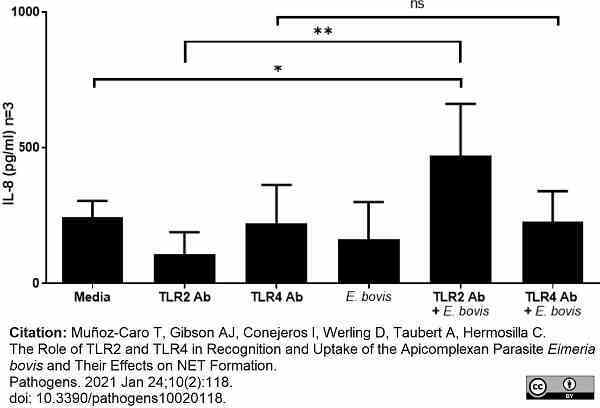
Muñoz-Caro, T. et al. (2021) The Role of TLR2 and TLR4 in Recognition and Uptake of the Apicomplexan Parasite Eimeria bovis and Their Effects on NET Formation.
Pathogens. 10 (2): 118. -
Mittal, P. et al. (2019) Outer kinetochore protein Dam1 promotes centromere clustering in parallel with Slk19 in budding yeast.
Chromosoma. 128 (2): 133-48.
- Synonyms
- CXCL8
- RRID
- AB_322152
- UniProt
- P36925
- Entrez Gene
- IL8
- GO Terms
- GO:0005153 interleukin-8 receptor binding
- GO:0005615 extracellular space
- GO:0006954 inflammatory response
- GO:0006955 immune response
- GO:0007050 cell cycle arrest
- GO:0008009 chemokine activity
- GO:0030155 regulation of cell adhesion
- GO:0030593 neutrophil chemotaxis
- GO:0045091 regulation of retroviral genome replication
- View More GO Terms
- GO:0050930 induction of positive chemotaxis
MCA1660
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Sheep ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up