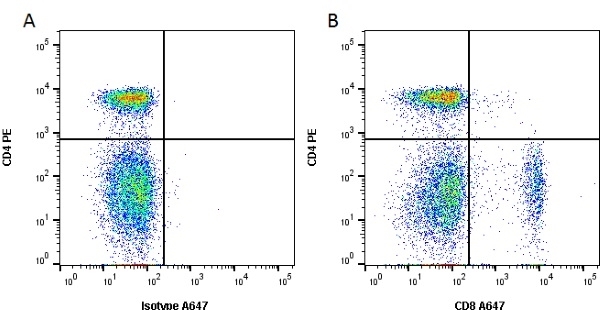
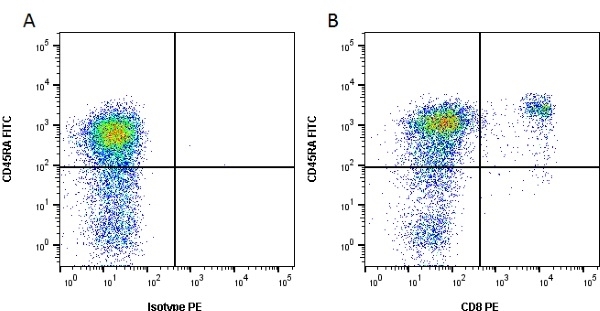
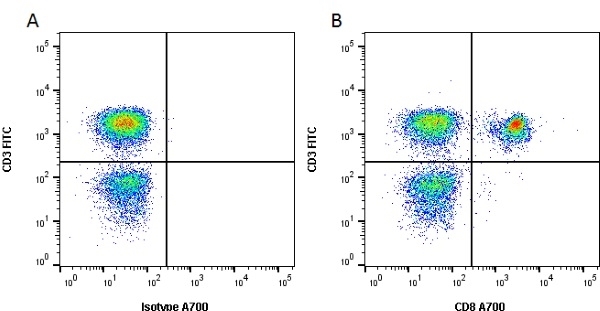
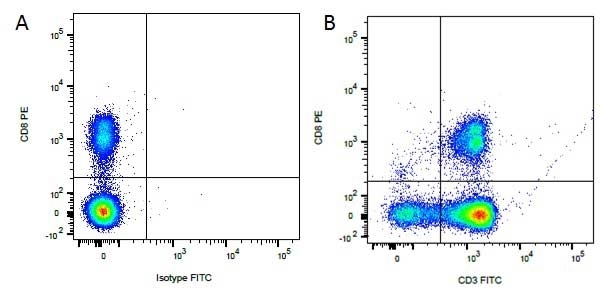
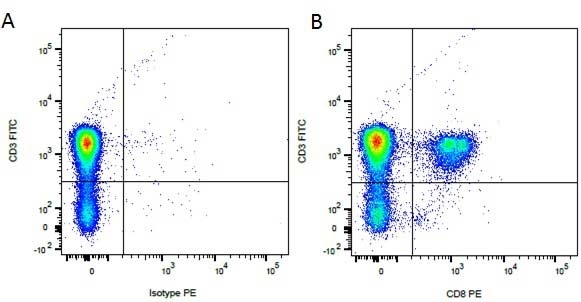
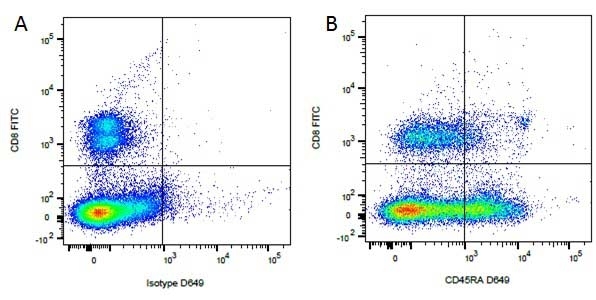
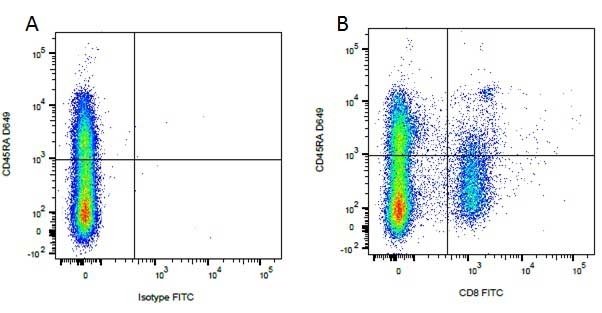
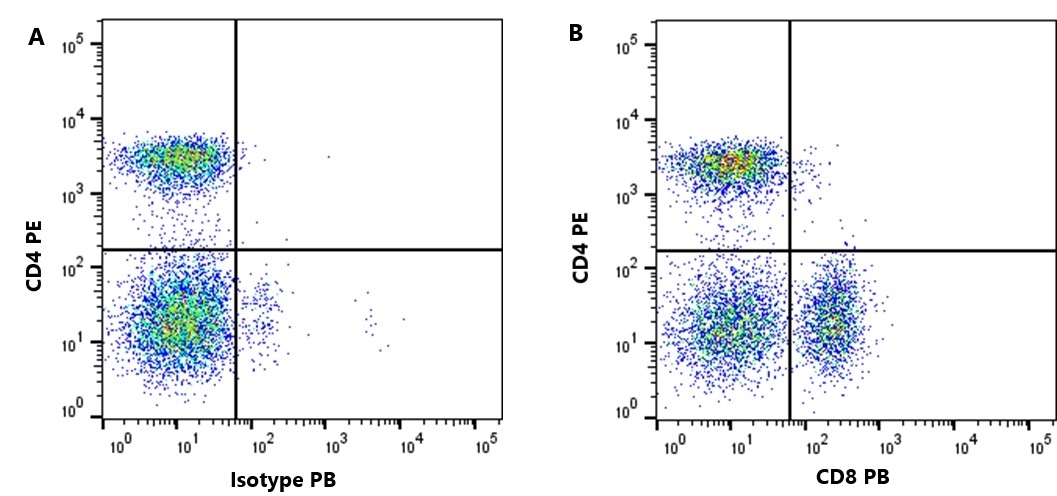
CD8 antibody | YCATE55.9












Rat anti Dog CD8
- Product Type
- Monoclonal Antibody
- Clone
- YCATE55.9
- Isotype
- IgG1
- Specificity
- CD8
| Rat anti Dog CD8 antibody, clone YCATE55.9 was clustered as Canine CD8 in the First Canine Leukocyte Antigen Workshop (Cobbold et al. 1994). YCATE55.9 reacts with a rat cell line transfected with cDNA for canine CD8α (Gorman et al. 1994) and blocks MHC class I dependant T-cell responses in vitro and in vivo. Rat anti Dog CD8, clone YCATE55.9 has been shown to deplete circulating CD8+ T cells when administered to dogs in vivo. (Watson et al. 1993) Reduced levels of circulating CD8+ T cells has been associated with decreased survival times for dogs with osteosarcoma (Biller et al. 2010). |
- Target Species
- Dog
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Carrier Free
- Yes
- Immunogen
- Canine CD8 alpha chimaeric human IgG1 Fc fusion protein.
- Approx. Protein Concentrations
- IgG concentration 1 mg/ml
- Fusion Partners
- Spleen cells from immunized DA rat were fused with cells of the Y3/Ag1.2.3 rat myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | 1/50 | 1/100 | |
| Immunofluorescence | |||
| Immunohistology - Frozen |
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 1 x 106 cells in 100μl
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Rat IgG1 Negative Control | MCA6004GA | F | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rat IgG1 Negative Control | ||||||
References for CD8 antibody
-
Cobbold, S. & Metcalfe, S. (1994) Monoclonal antibodies that define canine homologues of human CD antigens: summary of the First International Canine Leukocyte Antigen Workshop (CLAW).
Tissue Antigens. 43 (3): 137-54. -
Gorman, S.D. et al. (1994) Isolation and expression of cDNA encoding the canine CD4 and CD8 alpha antigens.
Tissue Antigens. 43 (3): 184-8. -
Watson, C.J. et al. (1993) CD4 and CD8 monoclonal antibody therapy: strategies to prolong renal allograft survival in the dog.
Br J Surg. 80 (11): 1389-92. -
Papadogiannakis, E.I. et al. (2009) Determination of intracellular cytokines IFN-gamma and IL-4 in canine T lymphocytes by flow cytometry following whole-blood culture.
Can J Vet Res. 73 (2): 137-43. -
Benyacoub, J. et al. (2003) Supplementation of food with Enterococcus faecium (SF68) stimulates immune functions in young dogs.
J Nutr. 133: 1158-62. -
Bird, R.C. et al. (2010) An autologous dendritic cell canine mammary tumor hybrid-cell fusion vaccine.
Cancer Immunol Immunother. 60: 87-97. -
Bund, D. et al. (2010) Canine-DCs using different serum-free methods as an approach to provide an animal-model for immunotherapeutic strategies.
Cell Immunol. 263: 88-98. -
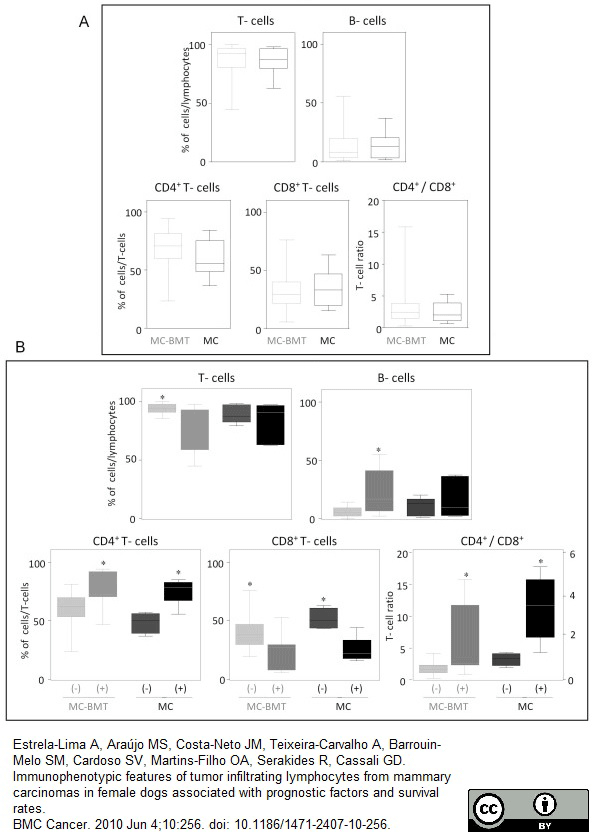
Estrela-Lima, A. et al. (2010) Immunophenotypic features of tumor infiltrating lymphocytes from mammary carcinomas in female dogs associated with prognostic factors and survival rates.
BMC Cancer. 10: 256.
View The Latest Product References
-
Huang, Y.C. et al. (2008) CD5-low expression lymphocytes in canine peripheral blood show characteristics of natural killer cells.
J Leukoc Biol. 84: 1501-10. -
Kornegay, J.N. et al. (2010) Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs.
Mol Ther. 18: 1501-8. -
Pichavant, C. et al. (2010) Expression of dog microdystrophin in mouse and dog muscles by gene therapy.
Mol Ther. 18: 1002-9. -
Pinheiro, D. et al (2011) Phenotypic and functional characterization of a CD4(+) CD25(high) FOXP3(high) regulatory T-cell population in the dog.
Immunology. 132: 111-22. -
Reis, A.B. et al. (2006) Phenotypic features of circulating leucocytes as immunological markers for clinical status and bone marrow parasite density in dogs naturally infected by Leishmania chagasi.
Clin Exp Immunol. 146: 303-11. -
Figueiredo, M.M. et al. (2014) Expression of Regulatory T Cells in Jejunum, Colon, and Cervical and Mesenteric Lymph Nodes of Dogs Naturally Infected with Leishmania infantum.
Infect Immun. 82: 3704-12. -
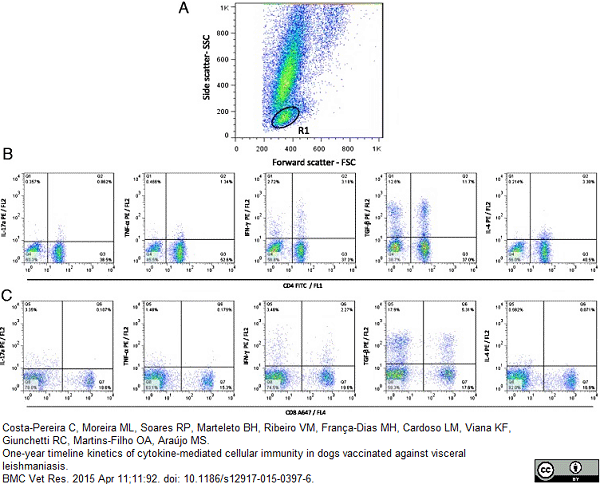
Costa-Pereira, C. et al. (2015) One-year timeline kinetics of cytokine-mediated cellular immunity in dogs vaccinated against visceral leishmaniasis.
BMC Vet Res. 11 (1): 92. -
Schaut, R.G. et al. (2016) Regulatory IgDhi B Cells Suppress T Cell Function via IL-10 and PD-L1 during Progressive Visceral Leishmaniasis.
J Immunol. 196 (10): 4100-9. -
Tagawa, M. et al. (2016) Evaluation of Costimulatory Molecules in Peripheral Blood Lymphocytes of Canine Patients with Histiocytic Sarcoma.
PLoS One. 11 (2): e0150030. -
Riondato, F. et al. (2016) Analytical and diagnostic validation of a flow cytometric strategy to quantify blood and marrow infiltration in dogs with large B-cell lymphoma.
Cytometry B Clin Cytom. 90 (6): 525-30. -
Cortese, L. et al. (2015) An immune-modulating diet increases the regulatory T cells and reduces T helper 1 inflammatory response in Leishmaniosis affected dogs treated with standard therapy.
BMC Vet Res. 11: 295. -
Miller, J. et al. (2015) Humoral and Cellular Immune Response in Canine Hypothyroidism.
J Comp Pathol. 153 (1): 28-37. -
Riondato, F. et al. (2016) Identification of a suitable internal control for fluorescence analysis on canine peripheral blood samples.
Vet Immunol Immunopathol. 172: 38-42. -
Martini, V. et al. (2016) Canine small clear cell/T-zone lymphoma: clinical presentation and outcome in a retrospective case series.
Vet Comp Oncol. 14 Suppl 1: 117-26. -
Gelain, M.E. et al. (2014) CD44 in canine leukemia: analysis of mRNA and protein expression in peripheral blood.
Vet Immunol Immunopathol. 159 (1-2): 91-6. -
Duz AL et al. (2014) The TcI and TcII Trypanosoma cruzi experimental infections induce distinct immune responses and cardiac fibrosis in dogs.
Mem Inst Oswaldo Cruz. 109 (8): 1005-13. -
Munhoz, T.D.et al. (2016) Regulatory T cells in dogs with multicentric lymphoma: peripheral blood quantification at diagnosis and after initial stage chemotherapy
Arq. Bras. Med. Vet. Zootec. 68 (1): 1-9. -
Bonnefont-Rebeix, C. et al. (2016) Characterization of a novel canine T-cell line established from a spontaneously occurring aggressive T-cell lymphoma with large granular cell morphology.
Immunobiology. 221 (1): 12-22. -
Viana, K.F. et al. (2015) Setting the proportion of CD4+ and CD8+ T-cells co-cultured with canine macrophages infected with Leishmania chagasi.
Vet Parasitol. 211 (3-4): 124-32. -
Bromberek, J.L. et al. (2016) Breed Distribution and Clinical Characteristics of B Cell Chronic Lymphocytic Leukemia in Dogs.
J Vet Intern Med. 30 (1): 215-22. -
Mie, K. et al. (2016) Influence of transfusion of lymphokine-activated T killer cells on inflammatory responses in dogs after laparotomy.
J Vet Med Sci. 78 (4): 579-85. -
Miglio, A. et al. (2014) Acute undifferentiated leukaemia in a dog.
Aust Vet J. 92 (12): 499-503. -
Villaescusa, A. et al. (2015) Effects of doxycycline on haematology, blood chemistry and peripheral blood lymphocyte subsets of healthy dogs and dogs naturally infected with Ehrlichia canis.
Vet J. 204 (3): 263-8. -
Fiuza JA et al. (2015) Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum.
Vaccine. 33 (2): 280-8. -
Perosso, J. et al. (2014) Alteration of sFAS and sFAS ligand expression during canine visceral leishmaniosis.
Vet Parasitol. 205 (3-4): 417-23. -
Heinrich, F. et al. (2015) Immunophenotyping of immune cell populations in the raccoon (Procyon lotor).
Vet Immunol Immunopathol. 168 (3-4): 140-6. -
Poggi, A. et al. (2017) Prognostic significance of Ki67 evaluated by flow cytometry in dogs with high-grade B-cell lymphoma.
Vet Comp Oncol. 15 (2): 431-40. -
McGill, J.L. et al. (2016) Vaccination with an Attenuated Mutant of Ehrlichia chaffeensis Induces Pathogen-Specific CD4+ T Cell Immunity and Protection from Tick-Transmitted Wild-Type Challenge in the Canine Host.
PLoS One. 11 (2): e0148229. -
Villaescusa, A. et al. (2012) Evaluation of peripheral blood lymphocyte subsets in family-owned dogs naturally infected by Ehrlichia canis.
Comp Immunol Microbiol Infect Dis. 35 (4): 391-6. -
Schaut RG et al. (2016) Recovery of antigen-specific T cell responses from dogs infected with Leishmania (L.) infantum by use of vaccine associated TLR-agonist adjuvant.
Vaccine. 34 (44): 5225-34. -
Miranda, S. et al. (2007) Characterization of circulating lymphocyte subpopulations in canine leishmaniasis throughout treatment with antimonials and allopurinol.
Vet Parasitol. 144 (3-4): 251-60. -
Viana, K.F. et al. (2016) Application of rapid in vitro co-culture system of macrophages and T-cell subsets to assess the immunogenicity of dogs vaccinated with live attenuated Leishmania donovani centrin deleted parasites (LdCen-/-).
Parasit Vectors. 9: 250. -
Michael, H.T. et al. (2013) Isolation and characterization of canine natural killer cells.
Vet Immunol Immunopathol. 155 (3): 211-7. -
Mitchell, L. et al. (2012) Induction of remission results in spontaneous enhancement of anti-tumor cytotoxic T-lymphocyte activity in dogs with B cell lymphoma.
Vet Immunol Immunopathol. 145 (3-4): 597-603. -
DaSilva, A.V.A. et al. (2018) Morphophysiological changes in the splenic extracellular matrix of Leishmania infantum-naturally infected dogs is associated with alterations in lymphoid niches and the CD4+ T cell frequency in spleens.
PLoS Negl Trop Dis. 12 (4): e0006445. -
Roatt, B.M.et al. (2017) A Vaccine Therapy for Canine Visceral Leishmaniasis Promoted Significant Improvement of Clinical and Immune Status with Reduction in Parasite Burden.
Front Immunol. 8: 217. -
Aricò, A. et al. (2013) The role of vascular endothelial growth factor and matrix metalloproteinases in canine lymphoma: in vivo and in vitro study.
BMC Vet Res. 9: 94. -
Aguiar-Soares, R.D.O. et al. (2020) Phase I and II Clinical Trial Comparing the LBSap, Leishmune®, and Leish-Tec® Vaccines against Canine Visceral Leishmaniasis.
Vaccines (Basel). 8 (4): 690. -
Martini, V. et al. (2019) Prognostic role of non-neoplastic lymphocytes in lymph node aspirates from dogs with diffuse large B-cell lymphoma treated with chemo-immunotherapy.
Res Vet Sci. 125: 130-5. -
Martins, G.C. et al. (2018) Clinical-pathological and immunological biomarkers in dogs with atopic dermatitis.
Vet Immunol Immunopathol. 205: 58-64. -
Anai, L.A. et al. (2017) Quantification of Treg cells in peripheral blood and lymph nodes of dogs with multicentric lymphoma
Arq. Bras. Med. Vet. Zootec. 69 (6): 1496-502. -
Wolf-Ringwall, A. et al. (2020) Prospective evaluation of flow cytometric characteristics, histopathologic diagnosis and clinical outcome in dogs with naïve B-cell lymphoma treated with a 19-week CHOP protocol.
Vet Comp Oncol. 18 (3): 342-52. -
Sayag, D. et al. (2020) Proof-of-concept study: Evaluation of plasma and urinary electrolytes as markers of response to L-asparaginase therapy in dogs with high-grade lymphoma.
Vet Clin Pathol. 49 (3): 476-83. -
Grudzien, M. et al. (2021) A newly established canine NK-type cell line and its cytotoxic properties.
Vet Comp Oncol. 19 (3): 567-77. -
Pellin, M.A. et al. (2017) Safety evaluation of combination doxorubicin and toceranib phosphate (Palladia®) in tumour bearing dogs: a phase I dose-finding study.
Vet Comp Oncol. 15 (3): 919-31. -
Kanei, T. et al. (2022) Expression and functional analysis of chemokine receptor 7 in canine lymphoma cell lines.
J Vet Med Sci. 84 (1): 25-30. -
Lee, S.H. et al. (2021) Safety and immunological effects of recombinant canine IL-15 in dogs.
Cytokine. 148: 155599. -
Knebel, A. et al. (2021) Measurement of canine Th17 cells by flow cytometry.
Vet Immunol Immunopathol. 243: 110366. -
do Prado Duzanski, A. et al. (2022) Cell-mediated immunity and expression of MHC class I and class II molecules in dogs naturally infected by canine transmissible venereal tumor: Is there complete spontaneous regression outside the experimental CTVT?
Research in Veterinary Science. 145: 193-204. -
Bragato, J.P. et al. (2022) miRNA-21 regulates CD69 and IL-10 expression in canine leishmaniasis.
PLoS One. 17 (3): e0265192. -
Riccardo, F. et al. (2022) Antigen mimicry as an effective strategy to induce CSPG4-targeted immunity in dogs with oral melanoma: a veterinary trial.
J Immunother Cancer. 10 (5): e004007. -
Troupel, T. et al. (2022) Generalised idiopathic polymyositis mimicking masticatory myositis in a dog
Vet Rec Case Rep. 10: e452. -
Konno, H. et al. (2022) An experimental challenge model for Leishmania donovani in beagle dogs, showing a similar pattern of parasite burden in the peripheral blood and liver.
Parasitol Res. 121 (12): 3569-79. -
Matralis, D.T. et al. (2023) Intracellular IFN-γ and IL-4 levels of CD4 + and CD8 + T cells in the peripheral blood of naturally infected (Leishmania infantum) symptomatic dogs before and following a 4-week treatment with miltefosine and allopurinol: a double-blinded, controlled and cross-sectional study.
Acta Vet Scand. 65 (1): 2. -
Hamouzová, P. et al. (2023) Lymphocyte immunophenotyping in dogs with lymphopenia of common causes.
Vet Immunol Immunopathol. 261: 110620. -
Bencze, M. et al. (2023) Receptor interacting protein kinase-3 mediates both myopathy and cardiomyopathy in preclinical animal models of Duchenne muscular dystrophy.
J Cachexia Sarcopenia Muscle.14 (6): 2520-31. -
Zwueste, D.M. et al. (2023) Oral cytarabine ocfosfate pharmacokinetics and assessment of leukocyte biomarkers in normal dogs.
J Vet Intern Med. 37 (6): 2429-42. -
Martini, V. et al. (2018) A retrospective study of flow cytometric characterization of suspected extranodal lymphomas in dogs.
J Vet Diagn Invest. 30 (6): 830-6. -
Lee, G.W. et al. (2021) Case Report: Long-Term Survival of a Dog With Chronic Lymphocytic Leukemia Treated With Chlorambucil, Prednisolone, and Imatinib.
Front Vet Sci. 8: 625527. -
Sainz, Á. et al. (2021) Effect of chemically modified tetracycline-8 (CMT-8) on hematology, blood chemistry, cytokines and peripheral blood lymphocyte subsets of healthy dogs.
Res Vet Sci. 136: 200-8. -
Hughes, K. et al. (2024) Canine T zone lymphoma is a tumor of mature, previously activated αβ T cells
Vet Immunol Immunopathol. 110725. -
Anthonyraj, S. et al. (2024) Chicory root powder included as a prebiotic in different cereal-based diets for dogs: Influences on gut health, metabolic and immunological status
Bioactive Carbohydrates and Dietary Fibre. : 100414. -
Sheng, R. et al. (2023) Prognostic significance of CD25 expression in dogs with a noninvasive diagnosis of B-cell lymphoma treated with CHOP chemotherapy.
Vet Comp Oncol. 21 (1): 28-35. -
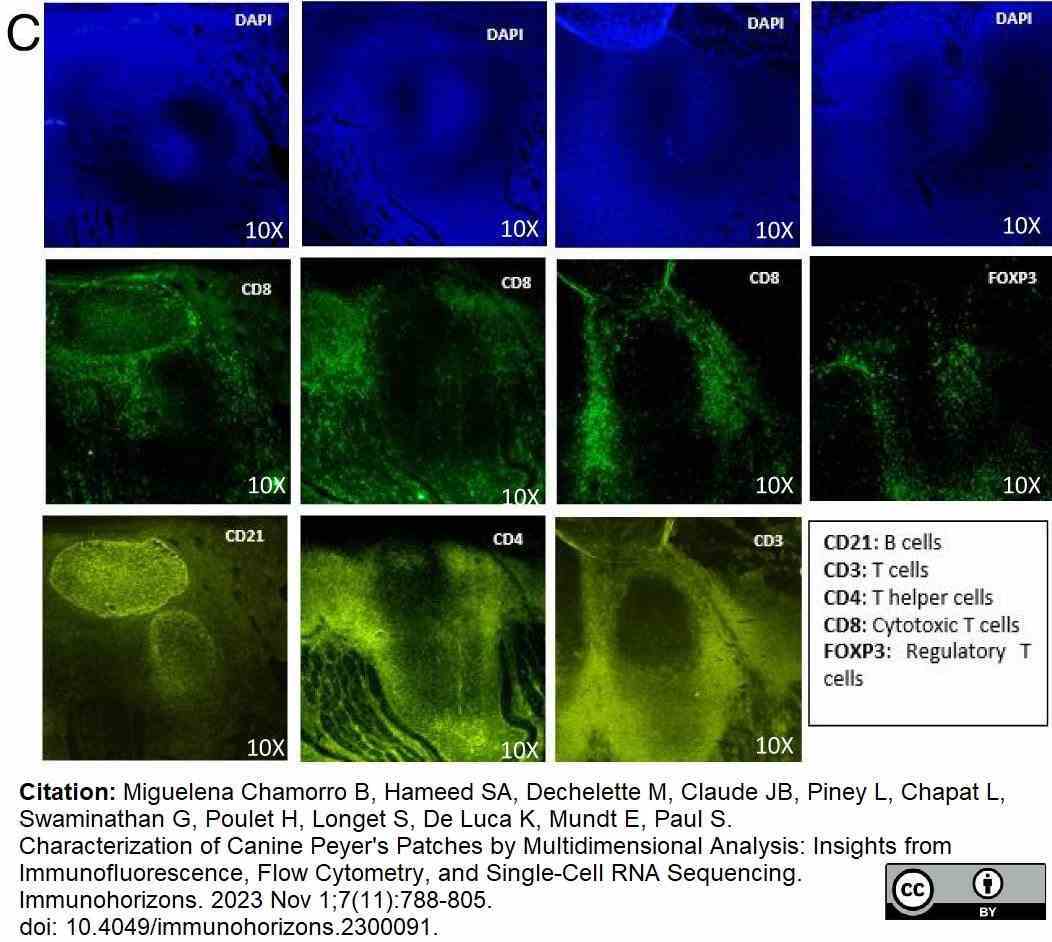
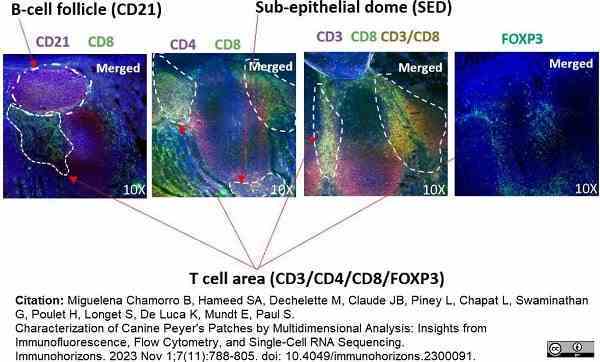
Miguelena Chamorro, B. et al. (2023) Characterization of Canine Peyer's Patches by Multidimensional Analysis: Insights from Immunofluorescence, Flow Cytometry, and Single-Cell RNA Sequencing.
Immunohorizons. 7 (11): 788-805.
- UniProt
- P33706
- Entrez Gene
- CD8A
- GO Terms
- GO:0016021 integral to membrane
MCA1039GA
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Dog ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up