-
US | en
- Clinical Diagnostics
- Endocrinology Antibodies
- Fertility Related Markers
- Inhibin Alpha Antibodies
- Inhibin and Activin Mini-review

Inhibin and Activin Mini-review
Inhibin and activin are part of the transforming growth factor beta (TGF-ß) family of cytokines (Bartholin et al. 2002), and are best known for their role in regulating follicle stimulating hormone (FSH) secretion, where inhibin inhibits and activin activates secretion. These protein regulators play a vital role in regulating FSH, which acts synergistically with luteinizing hormone (LH) in reproduction.
Activins
Activins are homodimers or heterodimers of disulfide linked ß subunits (ßA, ßB, ßC, ßD, and ßE) (Muenster et al. 2011). Activin A (ßAßA) and activin B (ßBßB) are the most studied subunits (Fig. 1). They were originally discovered in ovarian fluid and found to stimulate the biosynthesis and secretion of FSH from pituitary cells (Watanabe, 2011). Activins have since been found to be involved in several important biological activies, such as the tissue development and functions of pancreatic endocrine cells (Watanabe, 2011). Depending on their subunit composition, they also have an influence in the induction of apoptosis, metabolism, endocrine, homeostasis, bone growth, fibrosis inflammation, neurogenesis, tubulogenesis of endothelial cells, and carcinogenesis in several organs (Muenster et al. 2011, Chng et al. 2011). The importance of activin to a wide variety of human physiological mechanisms makes it an important drug target (Han, 2011). When activin is secreted its availability to be active and bind to its receptors can be blocked by other activin-binding proteins such as inhibin and follistatin (Gressner, 2011). Extracellular regulation of activin is achieved using follistatin and follistatin-related proteins (FLRP). Follistatin is also an activin binding protein, it binds directly to activin with high affinity and this stops activin from binding to its own receptors which blocks signaling (Bartholin et al. 2002). FLRP also binds to activin but with low reversibility. Its structural differences to follistatin allow it to act as a freely circulating activin-binding protein, but one which is not inhibitory to FSH as an autocrine agent7.
Inhibins
Inhibin alpha, encoded by the human INHA gene, has the opposite effect of activin. It also inhibits the biosynthesis and secretion of FSH, acting as an antagonist to activin (Gressner, 2011). Inhibins can be found in a wide range of tissues, and although they were originally found in the ovary (granulosa cells) and testis (sertoli cells), they have also been found in the brain and placenta. Inhibin has a common a-subunit which is attached to either a ßA or a ßB subunit via a disulfide bond to create inhibin A (a ßA) or inhibin B (a ßB) (Fig. 1). Inhibin is able to inhibit the activity of activin by competing for the binding site on the activin receptor (ActRII) and through displacing activin from the activin-receptor complex. However, the affinity of inhibin for the ActRII receptor is 10 fold lower than activin, so additional help is given to inhibin via a betaglycan with high affinity for inhibin, which acts as a coreceptor with ActRII to strengthen its inhibition (Choi and Han 2011). Inhibin alpha is expressed in a range of tissues including the endometrium (Mylonas et al. 2004), brain (Uccella et al. 2000), adrenal gland (Mc Cluggage et al. 1998), testis (Bergh and Cajander, 1990) and ovary (Cuevas et al. 1986, Yamoto et al. 1992).
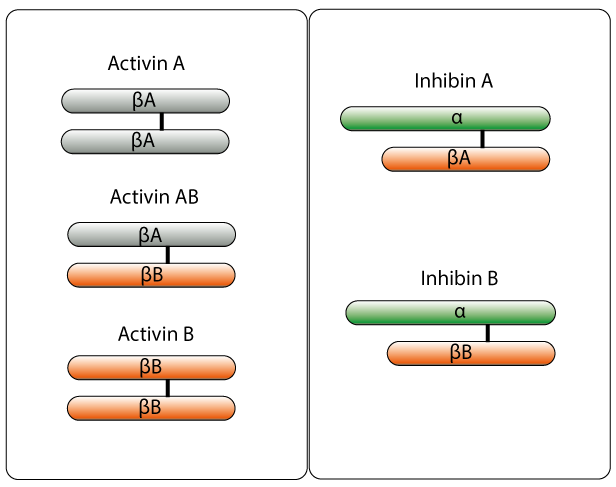
Fig. 1. Activin and inhibin homodimers showing the different combinations of the protein subunits.
Inhibin & Activin Resources
References
-
Bartholin L et al. (2002). Transcription activation of FLRG and follistatin by activin A, through Smad proteins, participates in a negative feedback loop to modulate activin A function.
Oncogene. 21(14): 2227-2235. -
Muenster U et al. (2011). Chapter Six - Antagonism of Activin by Activin Chimeras, In: Gerald Litwack, Editor(s).
Vitamins & Hormones Academic Press, 85: 105-128. -
Watanabe R (2011). Chapter One - Activin Receptor-Like Kinase and the Insulin Gene, In: Gerald Litwack, Editor(s).
Vitamins & Hormones, Academic Press, Volume 85, Pages 1-27. -
Chng Z et al. (2011). Chapter Three - Activin/Nodal Signaling and Pluripotency, In: Gerald Litwack, Editor(s).
Academic Press 85: 59-77. -
Han (2011). Chapter Two - Crystal Structure of Activin Receptor Type IIB Kinase Domain, In: Gerald Litwack, Editor(s).
Vitamins & Hormones, Academic Press, 85: 29-38. -
Gressner OA (2011). Chapter Four - Intracrine Signaling Mechanisms of Activin A and TGF-ß, In: Gerald Litwack, Editor(s)Vitamins & Hormones.
Vitamins & Hormones, Academic Press 85: 39-58. -
Choi S and Han J (2011). Chapter Five - Negative Regulation of Activin Signal Transduction, In: Gerald Litwack, Editor(s) Vitamins & Hormones.
Academic Press 85: 79-104. -
Mylonas I et al. (2004). Expression of the inhibin-alpha subunit in normal, hyperplastic and malignant endometrial tissue: an immunohistochemical analysis.
Gynecologic Oncology, 93(1):92-97. -
Uccella S et al. (2000). Localization of inhibin/activin subunits in normal pituitary adenomas.
Pituitary 3(3):131-9. -
Mc Cluggage WG et al. (1998). Immunihistochemical staining of normal, hyperplastic, and neoplastic adrenal cortex with a monoclonal antibody against alpha inhibin.
J. Clin. Pathol. 51(2):114-116. -
Bergh A and Cajander S (1990). Immunohistochemical localization of inhibin-a in the testes of normal men and in men with testicular disorders.
International Journal of Andrology, 13: 463–469. -
Cuevas P et al. (1986). Immunohistochemical detection of inhibin in the gonad.
Biochemical and Biophysical Research Communications. 142(1):23-30. -
Yamoto M et al. (1992). Immunohistochemical localization of inhibin/activin subunits in human ovarian follicles during the menstrual cycle.
The Journal of Clinical Endocrinology & Metabolism. 74(5):989-993.
