Immunohistochemistry and Staining of FFPE Tissues

- On This Page
- Overview
- Staining of FFPE tissues
- Related resources
- Support
Overview
Immunohistochemistry (IHC) is a common laboratory technique used to visualize proteins in tissue or tissue sections with the help of antibodies conjugated to enzymatic or fluorescent labels. For clarification of the terminology, it is important to differentiate this technique from the imaging of proteins in cells, a method referred to as immunocytochemistry (histo = tissue versus cyto = cell). Depending on how the immunostaining signal is visualized, the IHC method is classed as fluorescent or chromogenic IHC. In the past, chromogenic IHC was nearly exclusively performed, but recently due to increasing multiplexing needs (visualizing several proteins simultaneously in one tissue sample) fluorescent IHC is on the rise.
IHC is used from basic to clinical research, for example, to determine disease states and morphological abnormalities of human and animal tissues. Depending on how the tissue has been prepared, the IHC method is known as IHC-frozen (tissue has been snap frozen in liquid nitrogen, isopentane or dry ice) or IHC-paraffin (tissue has been fixed in formaldehyde and embedded in paraffin wax; FFPE). In both IHC-frozen and IHC-paraffin experiments the tissue or sections of the tissue are mounted on slides prior to staining. This is in contrast to the IHC-free-floating technique where the entire IHC procedure is performed in liquid to increase antibody binding and penetration; slide mounting only takes place upon experimental completion (McCollum no date). IHC-free-floating appears to be most popular in neuroscience research. When analysis of the tissue by electron microscopy is desired, the tissue is often embedded in acrylate resins such as glycol methacrylate (GMA), a technique referred to as IHC-resin (Howat et al. 2005).
Due to the significant differences in the described IHC procedures, it is essential to confirm that your primary antibody has been tested/validated in the intended IHC procedure before beginning your experiment. In addition to the availability of antibody reagents, your protein target itself may dictate the tissue preparation and IHC methods utilized. For example, for phosphoprotein visualization, dedicated preservation methods are required to conserve the post-translationally modified state before staining (Mueller et al. 2011).
Staining of FFPE Tissues
To support troubleshooting and confirm the specificity of the antibody staining, we recommend inclusion of both positive and negative controls in your experimental design. It is also critical to familiarize yourself with the expected staining pattern and your microscope set-up to ensure that you can detect the antibody staining.
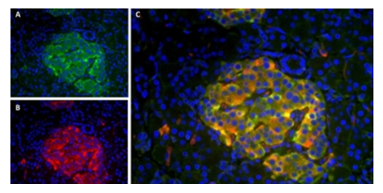
Fig. 1. Localization patterns of insulin and amylin in human pancreas section.
A, immunofluorescence staining of
paraffin embedded
human pancreas using Guinea Pig Anti-Pig Insulin Antibody (5330-0104G) and B, Mouse Anti-Human Amylin Antibody (MCA1126). C, Merged image showing co-localization of the two proteins. DAPI was used as nuclear counterstain (blue).
View 20-Step IHC-Paraffin Protocol Example
View Tips for Key Protocol Steps



