Human No Compensation Panels
Human peripheral blood panels
The no compensation panels below have been optimized for human peripheral blood to help identify common cell subsets. Useful as a simple four color panel or a great starting point when designing larger and more complex panels.
-
General identification panels
To identify T, B, NK and myeloid cells in human peripheral blood. -
T cell panels
To identify activated, memory and naïve subsets with helper and cytotoxic T cells. -
B cell panels
To identify naïve, memory, transitional and plasmablast B cells. -
NK cell and myeloid panels
To identify NK and NKT cells and various myeloid populations with peripheral blood.
General Identification Panels
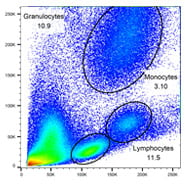
T, B and myeloid cells
| Markers | Formats | Catalog # |
|---|---|---|
| CD3 | RPE-Alexa Fluor 750 | MCA463P750 |
| CD19 | Pacific Blue | MCA1940PB |
| CD14 | Alexa Fluor 647 | MCA1568A647 |
| CD66b | FITC | MCA216F |
| Cells | Surface Markers |
|---|---|
| T cells | CD3+ CD19- CD14- CD66b- |
| B cells | CD3 - CD19 + CD14- CD66b- |
| Monocytes | CD3- CD19- CD14+ CD66b- |
| Granulocytes | CD3- CD19- CD14lo CD66b+ |
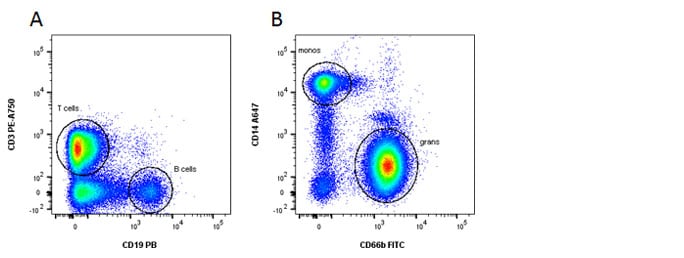
Fig. 1. General panel. A, Identification of lymphoid and B, myeloid cells using CD3, CD19, CD14 and CD66b

T, B and NK cells
| Markers | Formats | Catalog # |
|---|---|---|
| CD3 | FITC | MCA463F |
| CD45 | Pacific Blue | MCA87PB |
| CD19 | RPE-Alexa Fluor 750 | MCA1940P750 |
| CD16 / CD56 | Alexa Fluor 647 | MCA2537A647 / MCA2693A647 |
| Cells | Surface Markers |
|---|---|
| T cells | CD3+ CD19- CD45+ CD16- CD56- |
| B cells | CD3- CD19+ CD45+ CD16- CD56- |
| NK cells | CD3- CD19- CD45+ CD16+ CD56+ |
T Cell Panels

Helper and cytotoxic T cells
| Markers | Formats | Catalog # |
|---|---|---|
| CD3 | RPE-Alexa Fluor 750 | MCA463P750 |
| CD4 | Alexa Fluor 647 | MCA1267A647 |
| CD45 | Pacific Blue | MCA87PB |
| CD8 | FITC | MCA1226F |
| Cells | Surface Markers |
|---|---|
| Helper T cells | CD45+ CD3+ CD4+ CD8- |
| Cytotoxic T cells | CD45+ CD3+ CD4- CD8+ |

Activated T cells
| Markers | Formats | Catalog # |
|---|---|---|
| CD3 | Alexa Fluor 647 | MCA463A647 |
| CD4 or CD8 | Pacific Blue | MCA1267PB/MCA1226PB |
| CD38 | RPE-Alexa Fluor 750 | MCA1019P750 |
| HLA-DR | FITC | MCA71F |
| Cells | Surface Markers |
|---|---|
| Resting T helper cells | CD3+ CD4+ CD38- or HLA-DR- |
| Activated T helper cells | CD3+ CD4+ CD38+ or HLA-DR+ |
| Resting T cytotoxic cells | CD3+ CD8+ CD38- or HLA-DR- |
| Activated T cytotoxic cells | CD3+ CD8+ CD38+ or HLA-DR+ |
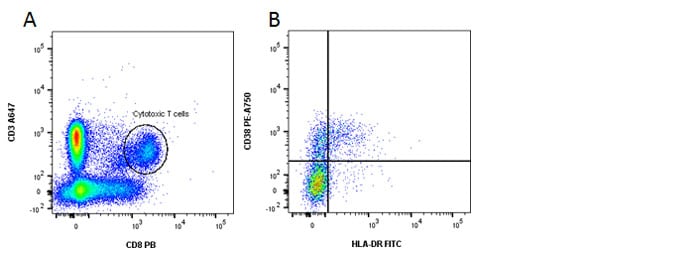
Fig. 2. Identification of activated CD8 T cells. A, CD3 and CD8 staining. B, HLA-DR and CD38 shows activated cells in the top right quadrant.

Memory and naïve T cells
| Markers | Formats | Catalog # |
|---|---|---|
| CD3 | RPE-Alexa Fluor 750 | MCA463P750 |
| CD4 / CD8 | Alexa Fluor 647 | MCA1267A647 or MCA1226A647 |
| CD45RO | FITC | MCA461F |
| CD45RA | Pacific Blue | MCA88PB |
| Cells | Surface Markers |
|---|---|
| Naïve T helper cells | CD3+ CD4+ CD45RA+ CD45RO- |
| Naïve T cytotoxic cells | CD3+ CD8+ CD45RA+ CD45RO- |
| Memory T helper cells | CD3+ CD4+ CD45RA- CD45RO+ |
| Memory T cytotoxic cells | CD3+ CD8+ CD45RA- CD45RO+ |
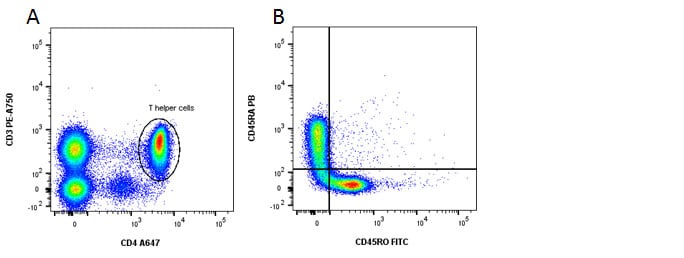
Fig. 3. Identification of memory and naïve CD4 T cells. A, CD3 and CD4 staining. B, CD45RA shows naïve cells in the top left and CD45RO shows memory cells in the bottom right quadrants.
Incorporating CD27 or CD62L into this panel will allow the identification of effector T cells.
| Cells | Surface Markers |
|---|---|
| Memory T helper cells | CD3+ CD4+ CD45RO+ CD27 and/or CD62L+ |
| Effector T helper cells | CD3+ CD4+ CD45RO- CD27 and/or CD62L- |
| Memory T cytotoxic cells | CD3+ CD8+ CD45RO+ CD27 and/or CD62L+ |
| Effector T cytotoxic cells | CD3+ CD8+ CD45RO- CD27 and/or CD62L- |

Treg cells (when permeabilizing is not required)
| Markers | Formats | Catalog # |
|---|---|---|
| CD3 | RPE-Alexa Fluor 750 | MCA463P750 |
| CD4 PB | Pacific Blue | MCA1267PB |
| CD25 | FITC | MCA2127F |
| Cells | Surface Markers |
|---|---|
| Treg cells | CD3+ CD4+ CD127lo CD25hi |
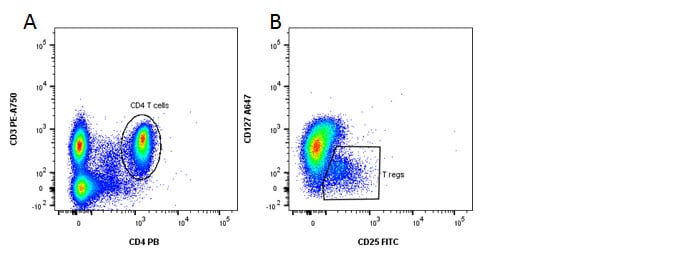
Fig. 4. Identification of regulatory T cells. A, CD3 and CD4 staining. B, CD127 and CD25 staining reveals the Treg population.
B Cell Panels

Memory and naïve B cells
| Markers | Formats | Catalog # |
|---|---|---|
| CD19 | RPE-Alexa Fluor 750 | MCA1940P750 |
| CD20 | Pacific Blue | MCA1710PB |
| IgD | FITC | STAR143F |
| CD27 | Alexa Fluor 647 | MCA755A647 |
| Cells | Surface Markers |
|---|---|
| Memory B cells | CD19+ CD20+ CD27+ IgD- |
| Naïve B cells | CD19+ CD20+ CD27- IgD+ |
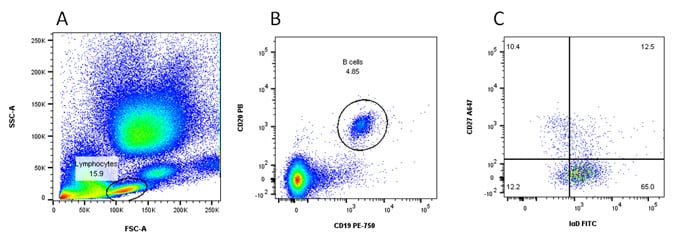
Fig. 5. Naïve and memory B cells. A, forward and side scatter. B, identification of lymphocyte population. C, naïve B cells can be seen in the bottom right and memory B cells in the top left quadrants.

Transitional B cells and plasmablasts
| Markers | Formats | Catalog # |
|---|---|---|
| CD19 | Alexa Fluor 647 | MCA1940A647 |
| CD20 | Pacific Blue | MCA1710PB |
| CD24 | FITC | MCA1379F |
| CD38 | RPE-Alexa Fluor 750 | MCA1019P750 |
| Cells | Surface Markers |
|---|---|
| Transitional B cells | CD19+ CD20+ CD24+ CD38+ |
| Plasmablasts | CD19+ CD20+ CD24- CD38+ |

Class switched memory B cells
| Markers | Formats | Catalog # |
|---|---|---|
| CD19 | RPE-Alexa Fluor 750 | MCA1940P750 |
| CD20 | Pacific Blue | MCA1710PB |
| CD27 | Alexa Fluor 647 | MCA755A647 |
| IgG | FITC | 204002F |
| Cells | Surface Markers |
|---|---|
| Non-class-switched memory B cells | CD19+ CD20+ CD27+ IgG- |
| Class-switched memory B cells | CD19+ CD20+ CD27+ IgG+ |
NK Cells and Myeloid Panels

NK and NKT cells
| Markers | Formats | Catalog # |
|---|---|---|
| CD3 | RPE-Alexa Fluor 750 | MCA463P750 |
| CD45 | Pacific Blue | MCA87PB |
| CD56 | Alexa Fluor 647 | MCA2693A647 |
| CD335 | FITC | MCA4646F |
| Cells | Surface Markers |
|---|---|
| T cells | CD3+, CD45+, CD56-, CD335- |
| NK cells | CD3-, CD45+, CD56+, CD335+ |
| NKT cells | CD3+, CD45+, CD56+ |
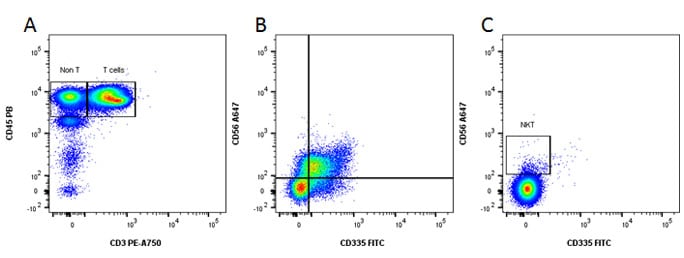
Fig. 6. Identification of NK and NKT cells. A, CD45 and CD3 staining. B, gated on the Non T, CD335 and CD56 staining reveals the NK population. C, gated on the T cell population reveals the NKT cells.

Myeloid cells
| Markers | Formats | Catalog # |
|---|---|---|
| CD14 | RPE-Alexa Fluor 750 | MCA1568P750 |
| CD66b | FITC | MCA216F |
| CD45 | Pacific Blue | MCA87PB |
| CD16 | Alexa Fluor 647 | MCA2537A647 |
| Cells | Surface Markers |
|---|---|
| Monocytes | CD45+ CD16+ CD14+ CD66b- |
| Granulocytes | CD45+ CD16+ CD14lo CD66b+ |
These no compensation panels are not available as pre-mixed four color panel cocktails and therefore may need to be optimized. Pre-optimized panels for human, mouse, rat or dog cell surface markers are available on our antibody panels and cocktails webpage. If you can’t find the antibody you are interested in, search our database of over 3000 flow cytometry tested antibodies.
To help you analyze your samples, information on the proportion of these populations in human blood can be found on our cell frequency page.
When performing multicolor flow cytometry, you should consider careful sample preparation, Fc blocking, inclusion of a live/dead dye, doublet exclusion and using the appropriate controls. For more information, watch our flow cytometry webinar Optimize your Flow Cytometry or visit our application resources page.









