Spectral Flow Cytometry with StarBright Dyes
Spectral Flow Cytometry Introduction
Spectral flow cytometry was first proposed in 1979 (Wade et al. 1979) and a first functional spectral flow cytometer presented at the International Society for Analytical Cytology in 2004 by J P Robinson (Robinson et al. 2004), with a patent issued to Purdue University in 2007 (Robinson 2019). Unlike conventional flow cytometry that partitions the emitted light from laser excited fluorophores onto specific detectors, spectral flow cytometry uses prisms to collect all the emitted light across an array of detectors. This allows capture of the entire spectral profile or signature from multiple lasers. The profiles of the fluorophores are then unmixed using complex mathematical models to separate and identify each fluorophore.
One advantage of spectral over conventional flow cytometry is the ability to use fluorophores with a similar peak emission, providing they have a distinct signature across the full spectrum. An example of this is APC and Alexa Fluor 647. These fluorophores cannot be used together in conventional flow cytometry, but the emission profile from the violet laser differs enough for them to be separated in spectral flow cytometry (Ferrer-Font et al. 2020). This technique allows the size of multicolor immunophenotyping panels to be extended to over 40 markers.
The same principles of good multicolor panel design are also required for spectral flow cytometry. Some knowledge of the biology of your experiment such as antigen density, the fluorophores, including brightness and optimization of your panel are still required. Ferrer-Font et al. (Ferrer-Font et al. 2020) have recently published in-depth information on panel design for spectral flow cytometry, Panel Design and Optimization for High-Dimensional Immunophenotyping Assays Using Spectral Flow Cytometry in Current Protocols in Cytometry.
Using StarBright Dyes in Spectral Flow Cytometry
New StarBright Dyes are proprietary, fluorescent nanoparticles developed specially for flow cytometry. They are bright with exacting excitation and emission characteristics. They can be used in all common staining buffers without the need for special buffers, have a high stability, and low lot-to-lot variation. We assessed their performance in a multicolor panel and found good separation of populations with expected percent positive (Figure 1). More multicolor panel data using full spectrum instruments can be found on our posters presented at AAI and CYTO.
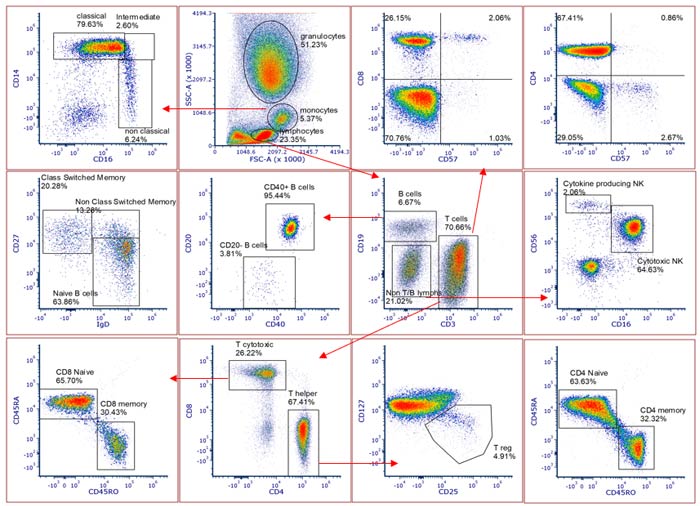
Fig 1. StarBright Dyes allow multiplexing in full spectrum flow cytometers. Human peripheral blood stained with CD14SV790 (MCA1568SBV790), CD16PE (MCA2537PE), CD19SBUV445 (MCA1940SBUV445), CD3SBUV400 (MCA463SBUV400), CD8SBV475 (MCA1226SBV475), CD4SBUV510 (MCA1267SBUV510), CD127A647, CD25SBV610 (MCA2127SBV610), CD45RAA700 (MCA88A700), CD45ROSBB700 (MCA461SBB700), CD56BV421, CD40APC-Cy7, IgDBV510, CD27SBV670 (MCA755SBV670) and CD57FITC analyzed on a full spectrum cytometer allowing identification of multiple common lineages and cell subsets. Axxx, Alexa Fluor; PE, phycoerythrin; SBB, StarBright Blue; SBUV, StarBright Ultraviolet; SBV, StarBright Violet.
Spectral Profiles of StarBright Dyes
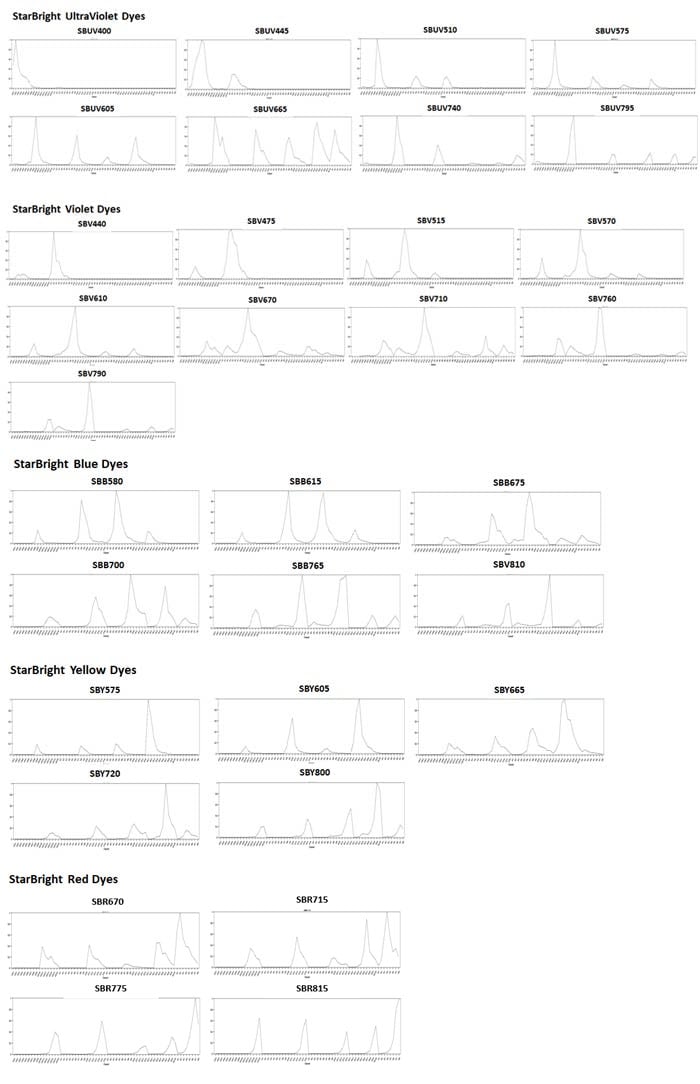
Fig. 2. Spectral profiles of StarBright Dyes. Spectral profiles of antibodies conjugated to StarBright Dyes, generated on a five-laser (16UV-16V-14B-10Y-8R) Aurora Spectral Analyzer (Cytek).
Benefits of StarBright Dyes in Spectral Flow Cytometry
One advantage of spectral flow cytometry is the ability to use fluorescent dyes with similar emission peaks together in a multicolor panel. This has led to the creation of 40+-color panels, whereas conventional flow cytometry is currently limited to around 30 colors. Many StarBright Dyes have a unique spectra, therefore novel combinations can be made with dyes that have similar maximal emission peaks. Examples of compatible dyes are StarBright Blue 700 (SBB700) Dye and PerCP-Cy5.5, StarBright Violet (SBV) 400 Dye with Brilliant Violet (BV) 421 and Pacific Blue, SBV475 with SBV515 and BV510, SBV610 and BV605, and SBV710 with BV711 (Figure 3).
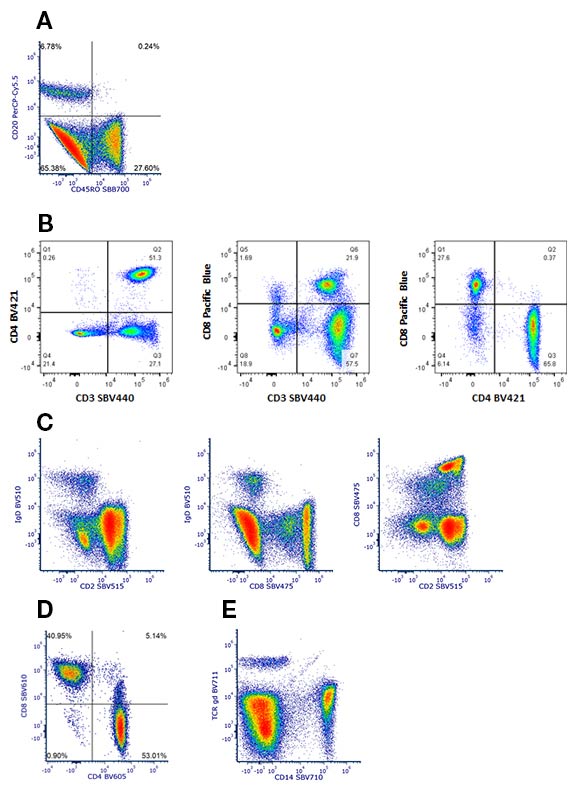
Fig. 3. Examples of novel dye combinations to stain human peripheral blood. A, CD20PerCP-Cy5.5 in combination with CD45ROSBB700 (MCA461SBB700), B, CD4BV421, CD8Pacific Blue (MCA1226PB), and CD3SBV440 (MCA463SBV440), C, IgDBV510, CD8SBV475 (MCA1226SBV475), and CD2SBV515 (MCA1194SBV515), D, CD4BV605 and CD8SBV610 (MCA1226SBV610), and E, TCR gdBV711 and CD14SBV710 (MCA1568SBV710).
Although many StarBright Dyes can be used with fluorescent dyes with similar maximal emission, enabling bigger panels to be created, many of the similarity scores are high, leading to excessive spreading. Therefore, best practice in panel design should always be followed. Separation of these dyes onto mutually exclusive markers can help negate the effect of the spreading. The tables below show the similarity scores of StarBright Dyes compared to Brilliant Ultraviolet (BUV), Brilliant Violet (BV), Brilliant Blue (BB), and PE and PE tandem dyes. change to The tables below show the similarity scores of StarBright Dyes compared to Brilliant Ultraviolet (BUV), Brilliant Violet (BV), Brilliant Blue (BB), PE and PE tandem, and APC and APC tandem dyes.
Table 1. StarBright Dye similarity scores.
StarBright UltraViolet Dye
StarBright UltraViolet Dye |
Comparison Dye |
Similarity Score |
|---|---|---|
|
BUV395 |
0.99 |
|
|
Dyl350 |
0.95 |
|
|
BUV496 |
0.96 |
|
|
BUV563 |
0.94 |
|
|
BUV615 |
0.91 |
|
|
BUV661 |
0.84 |
|
|
BUV737 |
0.94 |
|
|
BUV805 |
0.91 |
StarBright Violet Dye
StarBright Violet Dye |
Comparison Dye |
Similarity Score |
|---|---|---|
|
BV421 |
0.8 |
|
|
BV480 |
0.99 |
|
|
BV510 |
0.89 |
|
|
BV570 |
0.87 |
|
|
BV605 |
0.92 |
|
|
BV650 |
0.9 |
|
|
BV711 |
0.96 |
|
|
BV750 |
0.97 |
|
|
BV785 |
0.98 |
StarBright Blue Dye
StarBright Blue Dye |
Comparison Dye |
Similarity Score |
|---|---|---|
|
PE |
0.53 |
|
|
PE-Dazzle594 |
0.58 |
|
|
PerCP |
0.78 |
|
|
PerCP-Cy5.5/BB700 |
0.95/0.83 |
|
|
PE-Cy7/RB780 |
0.58/.0.75 |
|
|
PE-Cy7/RB780 |
0.56/0.87 |
StarBright Yellow Dye
StarBright Yellow Dye |
Comparison Dye |
Similarity Score |
|---|---|---|
|
PE/RY586 |
0.95/0.94 |
|
|
PE-Dazzle594 |
0.82 |
|
|
PE-Cy5 |
0.96 |
|
|
PE-Cy5.5 |
0.83 |
|
|
PE-Cy7 |
0.94 |
StarBright Red Dye
StarBright Yellow Dye |
Comparison Dye |
Similarity Score |
|---|---|---|
|
A647/APC |
0.85/0.93 |
|
|
A700/APC-R700 |
0.84/0.89 |
|
|
APC-Cy7 |
0.87 |
|
|
SBR815 |
APC-Fire810 |
0.82 |
Bio-Rad offer a full range of StarBright Dye conjugated antibodies. Mouse anti Human CD4 also available in 25 test sample sizes, discounts available with multiple purchases.
To help you build panels with StarBright Dyes, we have multiple posters presented at conferences available to download and/or listen to an on-demand webinar on how to expand your spectral flow cytometry knowledge and panel size.
Click on the links below to find out more about the benefits of StarBright Dyes and the range available for each laser line.
References
- Ferrer-Font et al. (Ferrer-Font et al. 2020). Panel Design and Optimization for High-Dimensional Immunophenotyping Assays Using Spectral Flow Cytometry. Current Protocols in Cytometry.
- Robinson JP et al. (2004). Collection hardware for high speed multispectral single particle analysis. Cytometry 59A, 12-12.
- Robinson JP (2019). Spectral flow cytometry — Quo vadimus? Cytometry Part A 95, 823-824.
- Wade CG et al. (1979). Spectra of cells in flow cytometry using a vidicon detector. Histochem Cytochem 27, 1049-52.
All trademarks used herein are the property of their respective owner.