Premixing StarBright Dyes
Problem
Preparing master mixes for large flow cytometry panels can be time-consuming, and if multiple samples are to be tested over a long period of time, there will be some differences in the master mixes resulting in errors. One way to avoid this is to prepare one master mix which can then be used over a long period of time.
However, how can you be assured that the premix will be stable over time?
Solution
Bio-Rad recently launched StarBright™ Dyes. These exceptionally bright dyes bring a new level of flexibility to your flow experiments, by allowing the resolution of rare populations and low-density antigens, without the requirement for special buffers even when multiplexing.
StarBright Dye conjugated antibodies can be premixed, with no loss of performance for up to six months.
The data below demonstrate this.
Proof of Principle
Following best practices, a small panel of four StarBright Dye conjugated antibodies, CD3 SBV610 (MCA463SBV610), CD4 SBB700 (MCA1267SBB700), CD8 SBV670 (MCA1226SBV670), and CD19 SBV515 (MCA1940SBV515), was designed. Antibodies were titrated before use and used in the panel at a concentration that gave the best stain index. We premixed these four StarBright Dyes and stored them as a master mix at 4oC for 28 days. We compared the staining of the freshly made master mix and the mix prepared 28 days before use on the same sample of peripheral blood. As you can see in Figure 1, the pattern of staining is very similar regardless of the age of the master mix, with similar B and T cell populations identified.
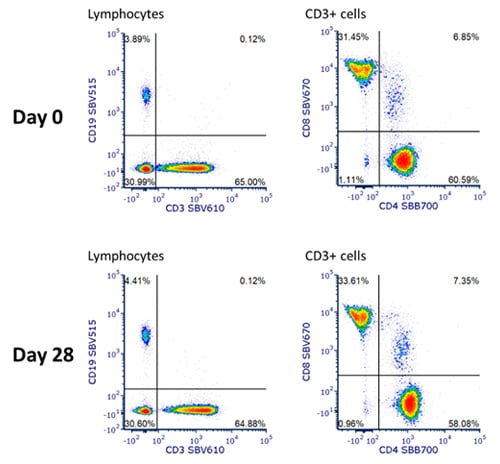
Fig.1. Staining of human blood with a premixed four StarBright Dye panel. Human peripheral blood was stained with an antibody mix consisting of CD3 SBV610 (MCA463SBV610), CD4 SBB700 (MCA1267SBB700), CD8 SBV670 (MCA1226SBV670), and CD19 SBV515 (MCA1940SBV515) made up fresh, or made with no additional storage buffer added, and kept at 4oC for 28 days. DRAQ7 was added just prior to acquiring on the ZE5 Cell Analyzer and cells were gated on live single cell lymphocytes.
Testing Storage Buffers
We also tested the effect of leaving the StarBright Dye mixes in three different buffers. We compared the samples stored in PBS (no buffer), Bio-Rad Staining Buffer (BUF073) which contains FBS, 1% BSA in PBS, and Brilliant Stain Buffer (BD), taking the staining volume to 50 µl/test. As can be seen from the graphs (Figure 2), apart from some minor variation when no buffer was used, there was very little difference in the staining over time, regardless of the buffer. The staining was very consistent and the same percent positive for each population could be identified.
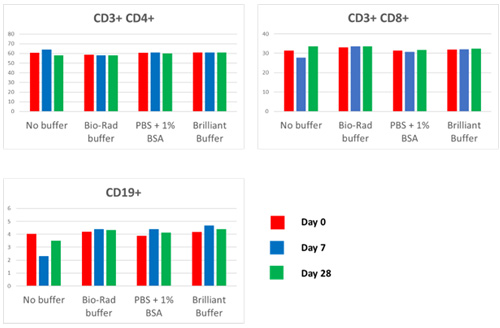
Fig. 2. Effect of different buffers on premixing. Graphs of percent positive for the CD3+, CD4+, CD3+, CD8+, and CD19+ populations, when StarBright Dye conjugated antibodies were stored in different buffers after premixing for up to 28 days.
Expanding the Panel Size
Once we had established the buffer had no effect on the premixed vs fresh staining profiles, we expanded the panel size and incubated the mix for 33 days at 4oC. By increasing the number of markers, we were able to detect more cell types. The new panel consisted of five StarBright UltraViolet and six StarBright Violet Dyes as shown in Table 1. Best practice in panel building was followed by titrating all antibodies prior to use, placing bright dyes on lower antigen density markers, and reducing the effect of spillover by placing those with high spillover on mutually exclusive markers.
Table 1. Antibodies used in premix staining panel to identify cell subsets in human peripheral blood.
Marker |
Fluorophore |
Cat. No |
|---|---|---|
|
CD20 |
SBUV400 |
|
|
CD4 |
SBUV510 |
|
|
CD3 |
SBUV605 |
|
|
CD19 |
SBUV665 |
|
|
CD45RO |
SBUV795 |
|
|
CD10 |
SBV440 |
|
|
CD33 |
SBV515 |
|
|
CD45RA |
SBV610 |
|
|
CD8 |
SBV670 |
|
|
CD14 |
SBV710 |
|
|
HLA DP DQ DR |
SBV790 |
As can be seen in Figure 3, the pattern of staining is very similar between the two master mixes, with similar T cell, B cell, monocyte, and granulocyte populations identified. Furthermore, as can be seen in the NxN two-color dot plots showing the staining of all lymphocyte markers (Figure 4) there was identical staining, with no interactions between the StarBright Dyes regardless of whether the panel was made fresh or incubated at 4oC for 33 days.
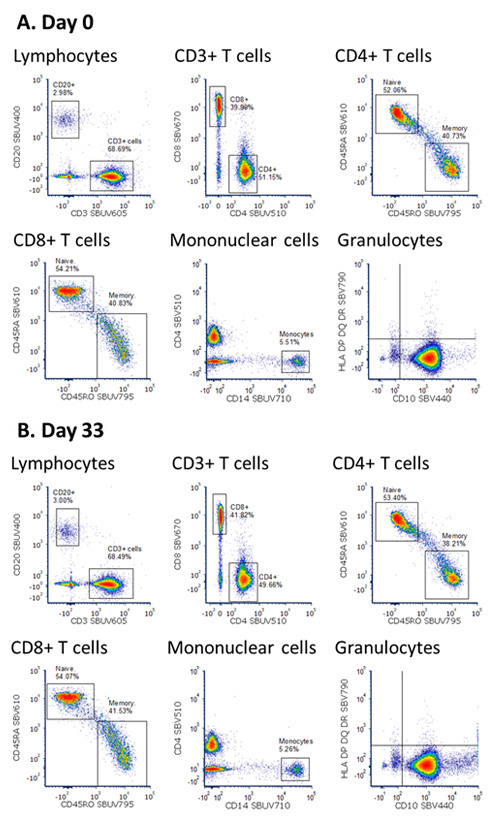
Fig. 3. Staining of human blood with a premixed StarBright Dye panel. Human peripheral blood was stained with an antibody mix consisting of StarBright UltraViolet and StarBright Violet Dyes with no additional storage buffer. DRAQ7 was added just prior to acquiring on the ZE5 Cell Analyzer. Cells were gated on live single cells and plots shown after staining with an antibody master mix prepared fresh or kept at 4oC for 33 days.
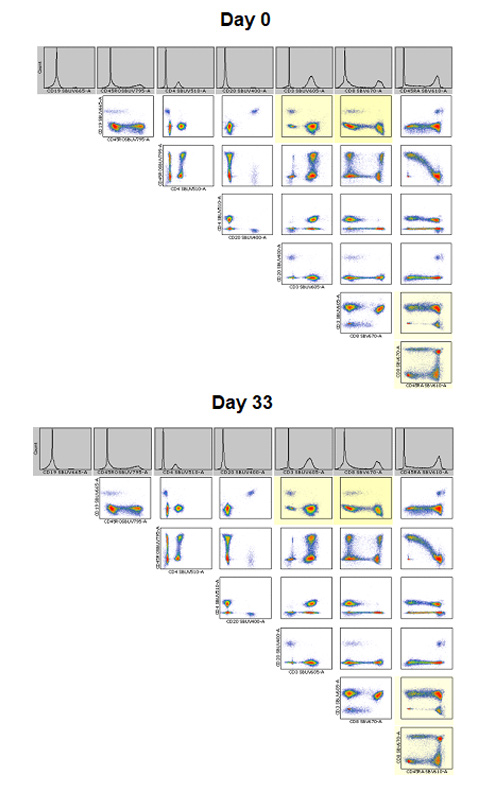
Fig 4. NxN plots of StarBright Dyes staining lymphocytes in the panel. NxN plots showing all of the two-color dot plot combinations for all StarBright Dye conjugated antibody staining of live single cell lymphocytes for the freshly stained and 33 day premixed panel.
Extended Storage Time
For some experiments, there may be a need to store the premix for a longer period. An example is in clinical studies when the same panel is used to stain multiple samples obtained over an extended period. Here we show that StarBright Dyes can be stored in a premixed panel at 4oC for up to six months. The panel contains nine antibodies conjugated to StarBright Dyes, as shown in Table 2. All antibodies were titrated prior to use in the panel.
Table 2. Antibodies used in the six month premixed staining panel to identify cell subsets in human peripheral blood.
Marker |
Fluorophore |
Cat. No |
|---|---|---|
|
CD14 |
SBUV400 |
|
|
CD45RA |
SBUV665 |
|
|
CD19 |
SBV440 |
|
|
CD45RO |
SBV610 |
|
|
CD4 |
SBB580 |
|
|
CD33 |
SBB675 |
|
|
CD8 |
SBB810 |
|
|
CD20 |
SBY575 |
|
|
CD3 |
SBY720 |
The premix was tested at regular intervals over the six month period by comparing the staining pattern on human peripheral blood with a freshly made master mix. The final time point at six months showed little variation in staining between the two panels, as shown in Figure 5.
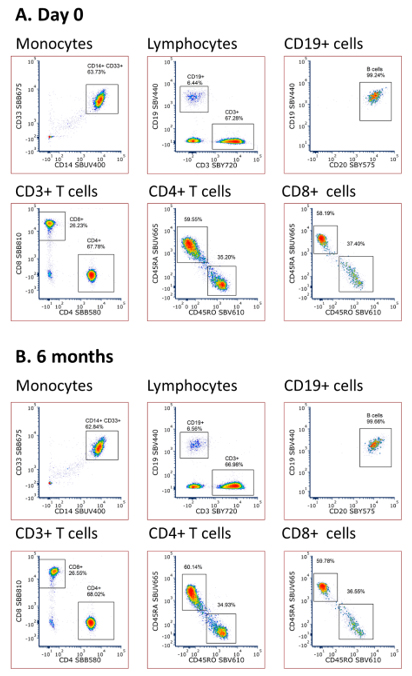
Fig 5. Staining of human blood with a fresh and six month premixed StarBright Dye panel. Human peripheral blood was stained with antibodies conjugated to StarBright UltraViolet, StarBright Violet, StarBright Blue, and StarBright Yellow Dyes. Propidium iodide was added just prior to acquiring on the ZE5 Cell Analyzer. Cells were gated on live single cells.
We have demonstrated that multiple StarBright Dyes are stable in a premixed panel for up to six months, when stored at 4°C. There is no significant impact on staining patterns compared to a freshly made panel.
Premixing your master mixes for multicolor panels allows you to save time, and money and reduce errors with no effect on the final data.
Visit our dedicated StarBright Dyes page to find out more about the dyes and antibodies available for use in your experiments.




