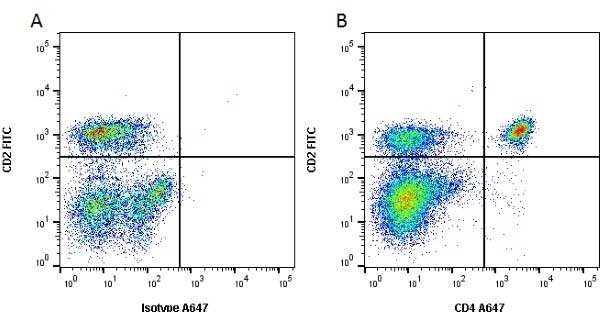
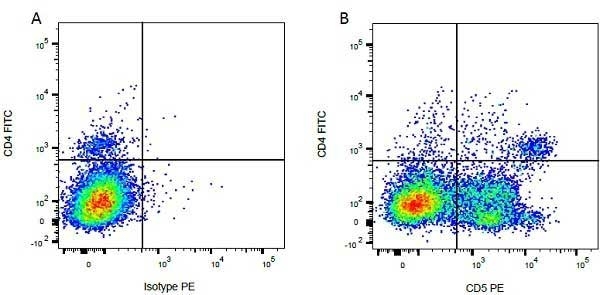
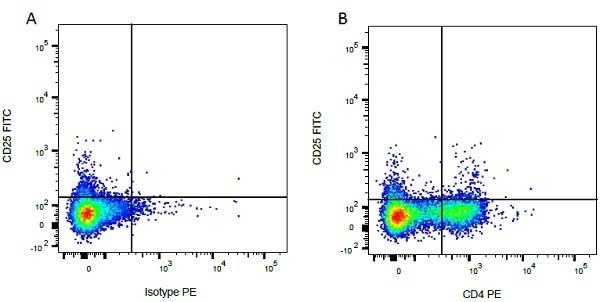
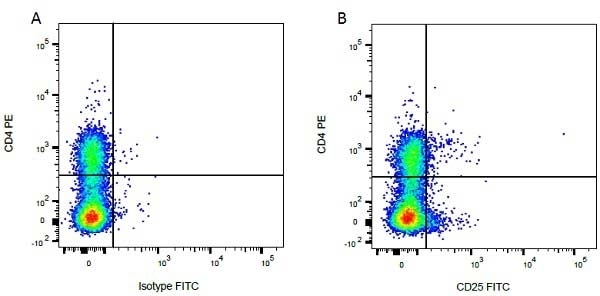
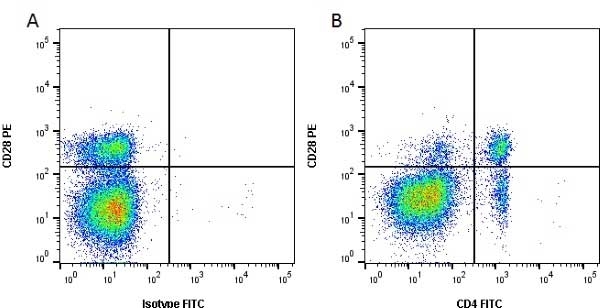
CD4 antibody | CC8

















Mouse anti Bovine CD4:FITC
- Product Type
- Monoclonal Antibody
- Clone
- CC8
- Isotype
- IgG2a
- Specificity
- CD4
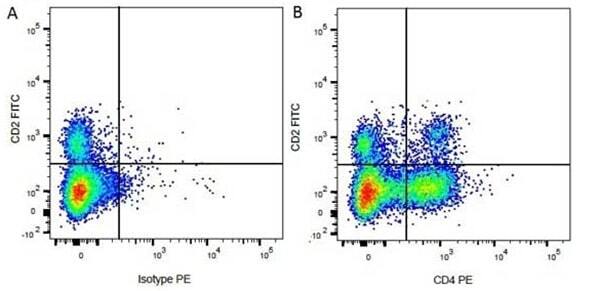
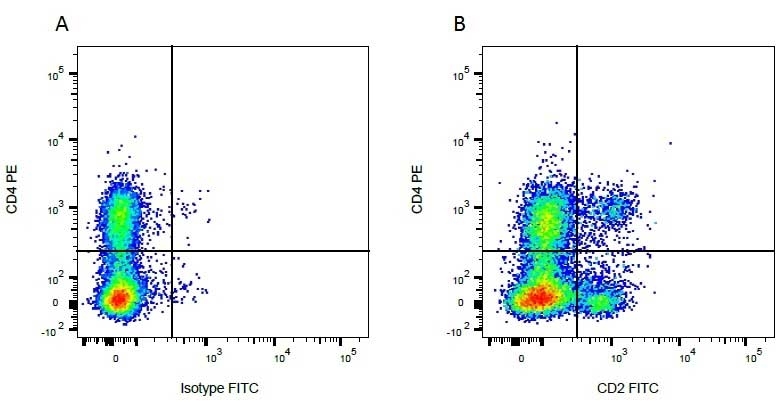
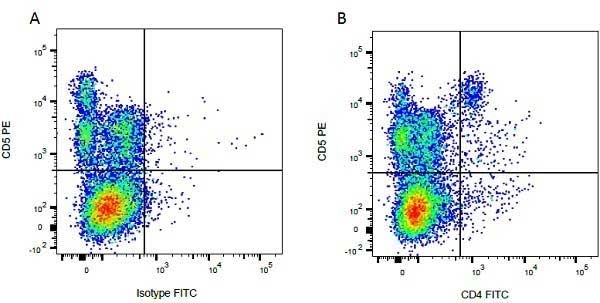
| Mouse anti Bovine CD4 antibody, clone CC8 recognizes bovine CD4, the homolog of human CD4 and immunoprecipitates a ~50 kDa molecule. The phenotype, tissue distribution and function of T-cells expressing the bovine CD4 antigen are similar to those in other species. However, expression on macrophages has not yet been detected. Mouse anti Bovine CD4 antibody, clone CC8 has been reported as being suitable for use on formalin dichromate (FD5) fixed paraffin embedded tissue with amplification and antigen retrieval techniques (Eskra et al. 1991). A mutation in the bovine CD4 gene resulting in an amino acid substitution at A324 T, located in the D4 domain of the CD4 gene product can occur. This mutation results in lowered binding of Mouse anti Bovine CD4 antibody, clone CC8 to CD4 in Japanese Black (JB) cattle where this mutation has been identified (Kato-Mori, et al.. 2020). CD4 in JB cattle can be identified using clone CACT138A (MCA6081) whose binding to bovine CD4 is unaffected by the A324T mutation (Kato-Mori, et al.. 2020). |
- Target Species
- Bovine
- Product Form
- Purified IgG conjugated to Fluorescein Isothiocyanate Isomer 1 (FITC) - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
1% bovine serum albumin - Immunogen
- Bovine lymphocytes.
- Approx. Protein Concentrations
- IgG concentration 0.1 mg/ml
- Fusion Partners
- Spleen cells from an immunized mouse were fused with cells of the mouse NS1 myeloma cell line.
- Max Ex/Em
-
Fluorophore Excitation Max (nm) Emission Max (nm) FITC 490 525 - Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended. This product is photosensitive and should be protected from light.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | Neat |
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells in 100μl
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG2a Negative Control:FITC | MCA929F | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG2a Negative Control:FITC | ||||||
References for CD4 antibody
-
Bensaid, A. & Hadam, M. (1991) Individual antigens of cattle. Bovine CD4 (BoCD4).
Vet Immunol Immunopathol. 27 (1-3): 51-4. -
Eskra, L. et al. (1991) Effect of monoclonal antibodies on in vitro function of T-cell subsets.
Vet Immunol Immunopathol. 27 (1-3): 215-22. -
Howard, C.J. et al. (1991) Summary of workshop findings for leukocyte antigens of cattle.
Vet Immunol Immunopathol. 27 (1-3): 21-7. -
Gutierrez, M. et al. (1999) The detection of CD2+, CD4+, CD8+, and WC1+ T lymphocytes, B cells and macrophages in fixed and paraffin embedded bovine tissue using a range of antigen recovery and signal amplification techniques.
Vet Immunol Immunopathol. 71 (3-4): 321-34. -
Harris, J. et al. (2002) Expression of caveolin by bovine lymphocytes and antigen-presenting cells
Immunology. 105: 190-5. -
Kruger, E.F. et al. (2003) Bovine monocytes induce immunoglobulin production in peripheral blood B lymphocytes.
Dev Comp Immunol. 27 (10): 889-97. -
Buddle, B.M. et al. (2003) Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination.
Infect Immun. 71: 6411-9. -
Endsley, J.J. et al. (2004) Characterization of bovine homologues of granulysin and NK-lysin.
J Immunol. 173 (4): 2607-14.
View The Latest Product References
-
Brackenbury, L.S. et al. (2005) Identification of a cell population that produces alpha/beta interferon in vitro and in vivo in response to noncytopathic bovine viral diarrhea virus.
J Virol. 79: 7738-44. -
Sidders, B. et al. (2008) Screening of highly expressed mycobacterial genes identifies Rv3615c as a useful differential diagnostic antigen for the Mycobacterium tuberculosis complex.
Infect Immun. 76: 3932-9. -
Gerner, W. et al. (2009) Identification of major histocompatibility complex restriction and anchor residues of foot-and-mouth disease virus-derived bovine T-cell epitopes.
J Virol. 83: 4039-50. -
Hu, X.D. et al. (2009) Immunotherapy with combined DNA vaccines is an effective treatment for M. bovis infection in cattle
Vaccine. 27: 1317-22. -
Lynch, E.M. et al. (2010) Effect of abrupt weaning at housing on leukocyte distribution, functional activity of neutrophils, and acute phase protein response of beef calves.
BMC Vet Res. 6: 39. -
Coad, M. et al. (2010) Repeat tuberculin skin testing leads to desensitisation in naturally infected tuberculous cattle which is associated with elevated interleukin-10 and decreased interleukin-1 beta responses.
Vet Res. 41: 14. -
Kiku, Y. et al. (2010) Decrease in bovine CD14 positive cells in colostrum is associated with the incidence of mastitis after calving.
Vet Res Commun. 34: 197-203. -
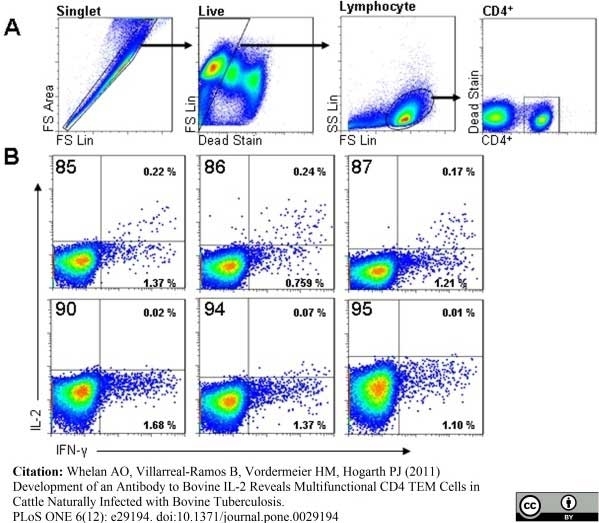
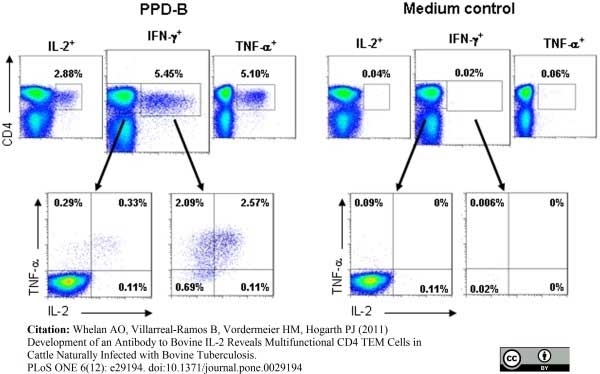
Whelan, A.O. et al. (2011) Development of an Antibody to Bovine IL-2 Reveals Multifunctional CD4 T(EM) Cells in Cattle Naturally Infected with Bovine Tuberculosis.
PLoS One. 6: e29194. -
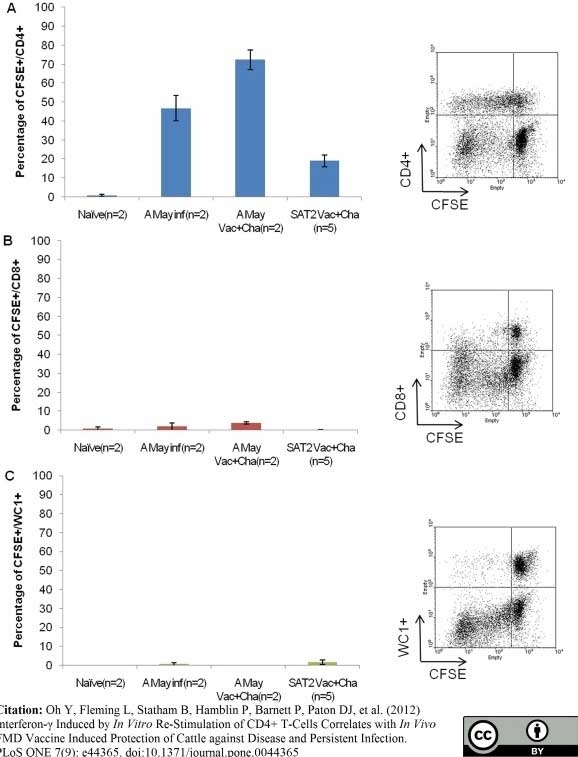
Oh, Y. et al. (2012) Interferon-γ induced by in vitro re-stimulation of CD4+ T-cells correlates with in vivo FMD vaccine induced protection of cattle against disease and persistent infection.
PLoS One. 7: e44365. -
Hine, B.C. et al. (2012) Analysis of leukocyte populations in Canadian Holsteins classified as high or low immune responders for antibody- or cell-mediated immune response.
Can J Vet Res. 76: 149-56. -
Aranday-Cortes, E. et al. (2012) Transcriptional profiling of disease-induced host responses in bovine tuberculosis and the identification of potential diagnostic biomarkers.
PLoS One. 7: e30626. -
Tenaya, I.W. et al. (2012) Flow cytometric analysis of lymphocyte subset kinetics in Bali cattle experimentally infected with Jembrana disease virus.
Vet Immunol Immunopathol. 149: 167-76. -
Wernike, K. et al. (2013) Oral exposure, reinfection and cellular immunity to Schmallenberg virus in cattle.
Vet Microbiol. pii: S0378-1135(13)00092-8. -
Dacal,. V. et al. (2013) Immunohistochemical characterization of inflammatory cells in the skin of cattle undergoing repeated infestations with Hypoderma lineatum (Diptera: Oestridae) larvae.
J Comp Pathol. 145: 282-8. -
Brodzki, P. et al. (2014) Phenotyping of leukocytes and granulocyte and monocyte phagocytic activity in the peripheral blood and uterus of cows with endometritis.
Theriogenology. 82 (3): 403-10. -
Grit, G.H. et al. (2014) Evaluation of cellular and humoral systemic immune response against Giardia duodenalis infection in cattle.
Vet Parasitol. 202 (3-4): 145-55. -
Blunt, L. et al. (2015) Phenotypic characterization of bovine memory cells responding to mycobacteria in IFN&gama; enzyme linked immunospot assays.
Vaccine. 33 (51): 7276-82. -
Okagawa, T. et al. (2016) Cooperation of PD-1 and LAG-3 Contributes to T-Cell Exhaustion in Anaplasma marginale-Infected Cattle.
Infect Immun. 84 (10): 2779-90. -
Metcalfe, H.J. et al. (2016) Protection associated with a TB vaccine is linked to increased frequency of Ag85A-specific CD4(+) T cells but no increase in avidity for Ag85A.
Vaccine. 34 (38): 4520-5. -
Diaz-San Segundo, F. et al. (2016) Combination of Adt-O1Manisa and Ad5-boIFNλ3 induces early protective immunity against foot-and-mouth disease in cattle.
Virology. 499: 340-9. -
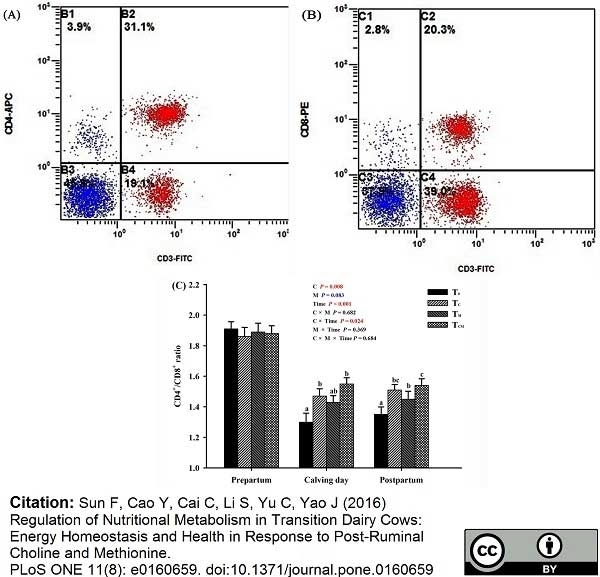
Sun, F. et al. (2016) Regulation of Nutritional Metabolism in Transition Dairy Cows: Energy Homeostasis and Health in Response to Post-Ruminal Choline and Methionine.
PLoS One. 11 (8): e0160659. -
Wattegedera, S.R. et al. (2017) Enhancing the toolbox to study IL-17A in cattle and sheep.
Vet Res. 48 (1): 20. -
Herry, V. et al. (2017) Local immunization impacts the response of dairy cows to Escherichia coli mastitis.
Sci Rep. 7 (1): 3441. -
Denholm, S.J. et al. (2017) Estimating genetic and phenotypic parameters of cellular immune-associated traits in dairy cows.
J Dairy Sci. 100 (4): 2850-62. -
Shimizu, T. et al. (2018) Changes of leukocyte counts and expression of pro- and anti-inflammatory cytokines in peripheral leukocytes in periparturient dairy cows with retained fetal membranes.
Anim Sci J. 89 (9): 1371-8. -
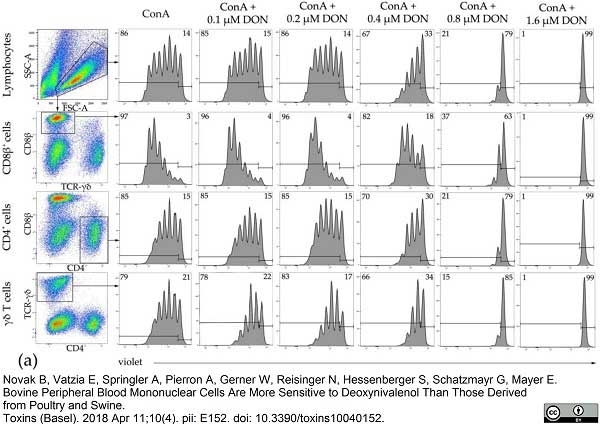
Novak, B. et al. (2018) Bovine Peripheral Blood Mononuclear Cells Are More Sensitive to Deoxynivalenol Than Those Derived from Poultry and Swine.
Toxins (Basel). 10 (4):152. -
Bassi, P.B. et al. (2018) Parasitological and immunological evaluation of cattle experimentally infected with Trypanosoma vivax.
Exp Parasitol. 185: 98-106. -
de Araújo, F.F. et al. (2019) Distinct immune response profile during Rhipicephalus (Boophilus) microplus infestations of guzerat dairy herd according to the maternal lineage ancestry (mitochondrial DNA).
Vet Parasitol. 273: 36-44. -
Nakajima, N. et al. (2019) Effects of direct exposure to cold weather under grazing in winter on the physiological, immunological, and behavioral conditions of Japanese Black beef cattle in central Japan.
Anim Sci J. 90 (8): 1033-41. -
Benedictus, L. et al. (2019) Immunization of young heifers with staphylococcal immune evasion proteins before natural exposure to Staphylococcus aureus induces a humoral immune response in serum and milk.
BMC Vet Res. 15 (1): 15. -
Grandoni, F. et al. (2019) A new polymorphic epitope of bovine CD4 antigen evidenced by flow cytometry.
Vet Immunol Immunopathol. 219: 109957. -
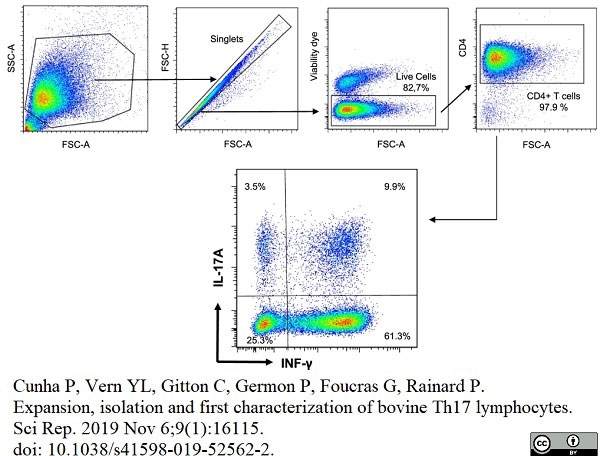
Cunha, P. et al. (2019) Expansion, isolation and first characterization of bovine Th17 lymphocytes.
Sci Rep. 9 (1): 16115. -
Risalde, M.A. et al. (2020) BVDV permissiveness and lack of expression of co-stimulatory molecules on PBMCs from calves pre-infected with BVDV.
Comp Immunol Microbiol Infect Dis. 68: 101388. -
Okino, C.H. et al. (2020) A polymorphic CD4 epitope related to increased susceptibility to Babesia bovis. in Canchim calves.
Vet Immunol Immunopathol. 230: 110132. -
Brodzki, P. et al. (2020) Selected leukocyte subpopulations in peripheral blood and uterine washings in cows before and after intrauterine administration of cefapirin and methisoprinol.
Anim Sci J. 91 (1): e13306. -
Gondaira, S. et al. (2020) Immunosuppression in Cows following Intramammary Infusion of Mycoplasma bovis.
Infect Immun. 88 (3): e00521-19. -
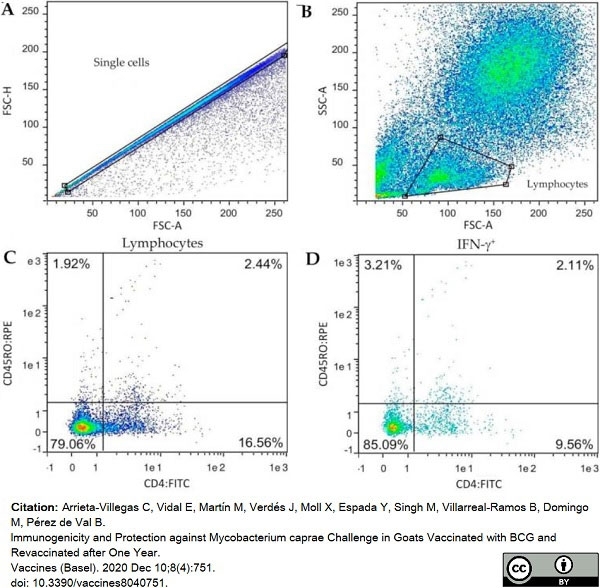
Arrieta-Villegas, C. et al. (2020) Immunogenicity and Protection against Mycobacterium caprae Challenge in Goats Vaccinated with BCG and Revaccinated after One Year.
Vaccines (Basel). 8 (4): 751. -
Bidart, J. et al. (2020) A New Cage-Like Particle Adjuvant Enhances Protection of Foot-and-Mouth Disease Vaccine.
Front Vet Sci. 7: 396. -
Colombatti, M.O. et al. (2021) Evaluation of a virulent strain of Mycobacterium avium subsp. paratuberculosis used as a heat-killed vaccine.
Vaccine. 39 (51): 7401-12. -
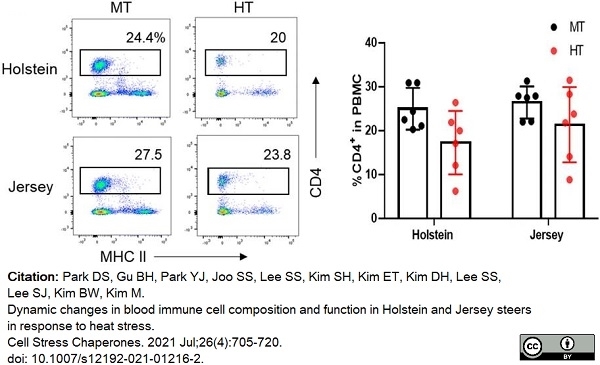
Park, D.S. et al. (2021) Dynamic changes in blood immune cell composition and function in Holstein and Jersey steers in response to heat stress.
Cell Stress Chaperones. 26 (4): 705-20. -
Kato-Mori, Y. et al. (2021) Characterization of a variant CD4 molecule in Japanese Black cattle.
Vet Immunol Immunopathol. 232: 110167. -
Okino, C.H. et al. (2022) CD4 bovine gene: Differential polymorphisms among cattle breeds and a new tool for rapid identification.
Vet Immunol Immunopathol. 251: 110462. -
Casaro, S. et al. (2022) Flow cytometry panels for immunophenotyping dairy cattle peripheral blood leukocytes
Vet immunol Immunopathol. 248: 110417. -
Hidalgo-Ruiz, M. et al. (2022) Babesia bovis AMA-1, MSA-2c and RAP-1 contain conserved B and T-cell epitopes, which generate neutralizing antibodies and a long-lasting Th1 immune response in vaccinated cattle.
Vaccine. S0264-410X(22)00049-4. -
Elsayed, M.S.A.E. et al. (2022) Real-time PCR using atpE, conventional PCR targeting different regions of difference, and flow cytometry for confirmation of Mycobacterium bovis. in buffaloes and cattle from the Delta area of Egypt.
BMC Microbiol. 22 (1): 154. -
Tucker, N. et al. (2023) Bovine blood and milk T-cell subsets in distinct states of activation and differentiation during subclinical Staphylococcus aureus mastitis.
J Reprod Immunol. 156: 103826. -
Benedictus, L. et al. (2019) Immunization of young heifers with staphylococcal immune evasion proteins before natural exposure to Staphylococcus aureus induces a humoral immune response in serum and milk.
BMC Vet Res. 15 (1): 15. -
Yang, L. et al. (2018) Association of the expression of Th cytokines with peripheral CD4 and CD8 lymphocyte subsets after vaccination with FMD vaccine in Holstein young sires.
Res Vet Sci. 119: 79-84.
MCA1653F
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Bovine ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up