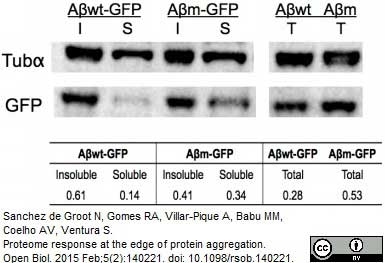
Tubulin Alpha antibody | YOL1/34

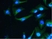
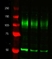








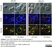

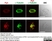
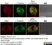




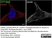
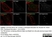
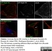




Rat anti Tubulin Alpha
- Product Type
- Monoclonal Antibody
- Clone
- YOL1/34
- Isotype
- IgG2a
- Specificity
- Tubulin Alpha
| Rat anti tubulin alpha antibody, clone YOL1/34 recognizes the alpha subunit of tubulin. The reactivity pattern is similar to that seen with clone YL1/2. |
- Target Species
- Yeast
- Species Cross-Reactivity
-
Target Species Cross Reactivity Birds Expected from Sequence Mammals Expected from Sequence Drosophila Fungal Expected from Sequence Human Arabidopsis thaliana Saccharomyces Platyzoma Ashbya Mouse Naegleria Asplenium nidus Seagrass (Cymodocea nodosa) Rye (Secale cereale L.) - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant.
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
0.09% Sodium Azide - Carrier Free
- Yes
- Immunogen
- Yeast tubulin.
- Approx. Protein Concentrations
- IgG concentration 1.0mg/ml
- Fusion Partners
- Spleen cells from immunized LOU rats were fused with cells of the rat YB2/0 myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA | 10ug/ml as detecting antibody | ||
| Immunofluorescence | |||
| Immunohistology - Frozen | |||
| Radioimmunoassays | |||
| Western Blotting |
Source Reference
-
Kilmartin, J.V. et al. (1982) Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line.
J Cell Biol. 93 (3): 576-82.
References for Tubulin Alpha antibody
-
Sullivan, M. et al. (2001) Orchestrating anaphase and mitotic exit: separase cleavage and localization of Slk19.
Nat Cell Biol. 3: 771-7. -
Trieselmann, N. et al. (2003) Ran modulates spindle assembly by regulating a subset of TPX2 and Kid activities including Aurora A activation.
J Cell Sci. 116: 4791-8. -
Petronczki, M. et al. (2004) Sister-chromatid cohesion mediated by the alternative RF-CCtf18/Dcc1/Ctf8, the helicase Chl1 and the polymerase-alpha-associated protein Ctf4 is essential for chromatid disjunction during meiosis II.
J Cell Sci. 117: 3547-59. -
Chatzimeletiou, K. et al. (2005) Spindle abnormalities in normally developing and arrested human preimplantation embryos in vitro identified by confocal laser scanning microscopy.
Hum Reprod. 20: 672-82. -
White, J. et al. (2005) Developmental activation of the Rb-E2F pathway and establishment of cell cycle-regulated cyclin-dependent kinase activity during embryonic stem cell differentiation.
Mol Biol Cell. 16 (4): 2018-27. -
Yu, H.G. et al. (2007) The Aurora kinase Ipl1 maintains the centromeric localization of PP2A to protect cohesin during meiosis.
J Cell Biol. 176: 911-8. -
D'Ambrosio, C. et al. (2008) Identification of cis-acting sites for condensin loading onto budding yeast chromosomes.
Genes Dev. 22: 2215-27 -
Sullivan, M. et al. (2008) Cyclin-specific control of ribosomal DNA segregation.
Mol Cell Biol. 28: 5328-36.
View The Latest Product References
-
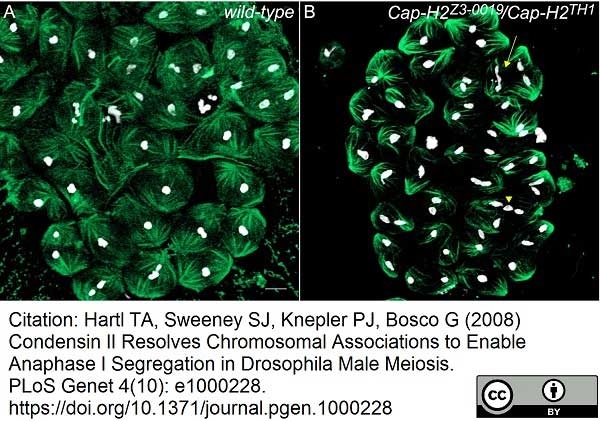
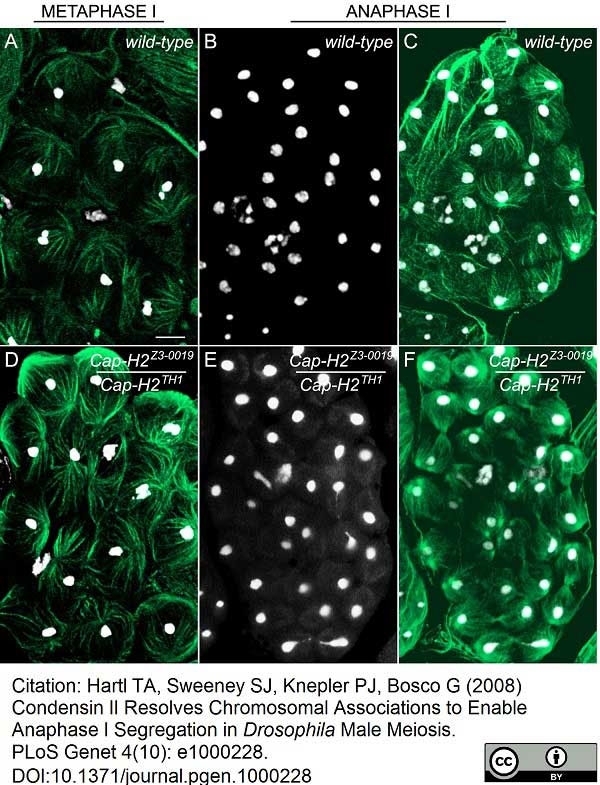
Hartl, T.A. et al. (2008) Condensin II resolves chromosomal associations to enable anaphase I segregation in Drosophila male meiosis.
PLoS Genet. 4: e1000228. -
Waples, W.G. et al. (2009) Putting the brake on FEAR: Tof2 promotes the biphasic release of Cdc14 phosphatase during mitotic exit.
Mol Biol Cell. 20 (1): 245-55. -
Fonseca, A.V. et al. (2010) Polarization and migration of hematopoietic stem and progenitor cells rely on the RhoA/ROCK I pathway and an active reorganization of the microtubule network.
J Biol Chem. 285: 31661-71. -
Lang, C. et al. (2010) Structural mutants of the spindle pole body cause distinct alteration of cytoplasmic microtubules and nuclear dynamics in multinucleated hyphae.
Mol Biol Cell. 21 (5): 753-66. -
Lang, C. et al. (2010) Mobility, microtubule nucleation and structure of microtubule-organizing centers in multinucleated hyphae of Ashbya gossypii.
Mol Biol Cell. 21: 18-28. -
Rossio, V. et al. (2010) The RSC chromatin-remodeling complex influences mitotic exit and adaptation to the spindle assembly checkpoint by controlling the Cdc14 phosphatase
J Cell Biol. 191: 981-97. -
Mirchenko, L. and Uhlmann, F. (2010) Sli15(INCENP) dephosphorylation prevents mitotic checkpoint reengagement due to loss of tension at anaphase onset.
Curr Biol. 20: 1396-401. -
Takeo, S. et al. (2010) Calcineurin and its regulation by Sra/RCAN is required for completion of meiosis in Drosophila.
Dev Biol. 344 (2): 957-67. -
Whelan, F. et al. (2010) Amino acid substitutions in the aryl hydrocarbon receptor ligand binding domain reveal YH439 as an atypical AhR activator.
Mol Pharmacol. 77: 1037-46. -
Finlayson, M.R. et al. (2011) Regulation of exit from mitosis in multinucleate Ashbya gossypii cells relies on a minimal network of genes.
Mol Biol Cell. 22 (17): 3081-93. -
Keeling, J.W. and Miller, R.K. (2011) Indirect immunofluorescence for monitoring spindle assembly and disassembly in yeast.
Methods Mol Biol. 782: 231-44. -
Raspelli, E. et al. (2011) Budding yeast Dma1 and Dma2 participate in regulation of Swe1 levels and localization.
Mol Biol Cell. 22: 2185-97. -
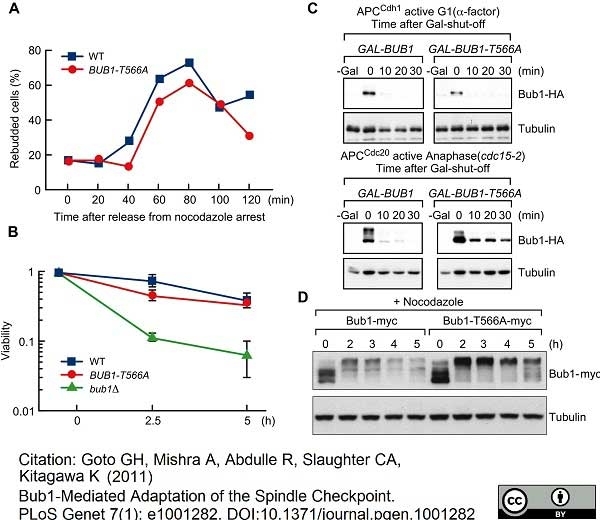
Goto, G.H. et al. (2011) Bub1-mediated adaptation of the spindle checkpoint.
PLoS Genet. 7: e1001282. -
Rossio, V. and Yoshida, S. (2011) Spatial regulation of Cdc55-PP2A by Zds1/Zds2 controls mitotic entry and mitotic exit in budding yeast.
J Cell Biol. 193: 445-54. -
Hao, N. et al. (2011) Identification of residues in the N-terminal PAS domains important for dimerization of Arnt and AhR.
Nucleic Acids Res. 39 (9): 3695-709. -
Grava, S. et al. (2011) Clustering of Nuclei in Multinucleated Hyphae Is Prevented by Dynein-Driven Bidirectional Nuclear Movements and Microtubule Growth Control in Ashbya gossypii.
Eukaryot Cell. 10: 902-15. -
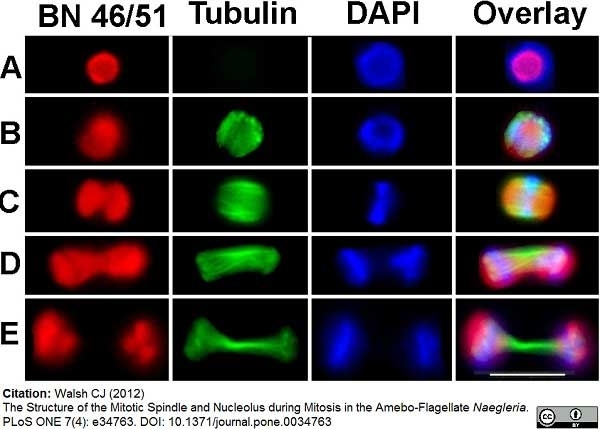
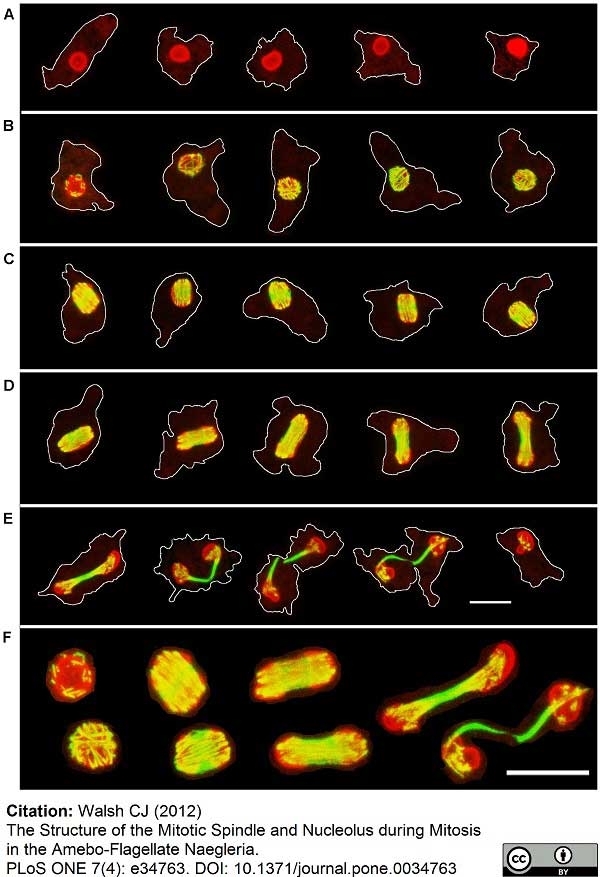
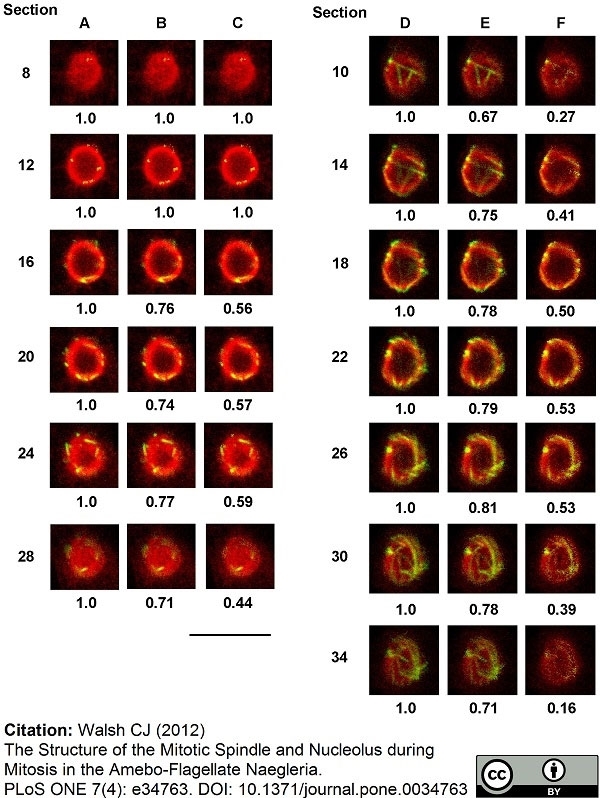
Walsh, C.J. (2012) The structure of the mitotic spindle and nucleolus during mitosis in the amebo-flagellate Naegleria.
PLoS One. 7: e34763. -
Chatzimeletiou, K. et al. (2012) Cytoskeletal analysis of human blastocysts by confocal laser scanning microscopy following vitrification.
Hum Reprod. 27: 106-13. -
Elhanany-Tamir, H. et al. (2012) Organelle positioning in muscles requires cooperation between two KASH proteins and microtubules.
J Cell Biol. 198 (5): 833-46. -
Malea, P. et al. (2013) Microtubule integrity and cell viability under metal (Cu, Ni and Cr) stress in the seagrass Cymodocea nodosa.
Chemosphere. pii: S0045-6535(13)00820-5. -
Panteris, E. et al. (2013) The distribution of TPX2 in dividing leaf cells of the fern Asplenium nidus.
Plant Biol (Stuttg). 15: 203-9. -
Buerstenbinder, K. et al. (2013) Arabidopsis Calmodulin-binding IQD1 Localizes to Microtubules and Interacts with Kinesin Light Chain-Related Protein-1.
J Biol Chem. 288: 1871-82. -
Adamakis, I.D. et al. (2013) Effects of bisphenol A on the microtubule arrays in root meristematic cells of Pisum sativum L.
Mutat Res. 750 (1-2): 111-20. -
Hao, N. et al. (2013) Reciprocal regulation of the basic helix-loop-helix/Per-Arnt-Sim partner proteins, Arnt and Arnt2, during neuronal differentiation.
Nucleic Acids Res. 41 (11): 5626-38. -
Wang, S. et al. (2015) Nesprin provides elastic properties to muscle nuclei by cooperating with spectraplakin and EB1.
J Cell Biol. 209 (4): 529-38. -
Eleftheriou, E.P. et al. (2015) Aberration of mitosis by hexavalent chromium in some Fabaceae members is mediated by species-specific microtubule disruption.
Environ Sci Pollut Res Int. 22 (10): 7590-9. -
Schweizer, N. et al. (2015) An organelle-exclusion envelope assists mitosis and underlies distinct molecular crowding in the spindle region.
J Cell Biol. 210 (5): 695-704. -
Bacon, T. et al. (2015) Histone deacetylase 3 indirectly modulates tubulin acetylation.
Biochem J. 472 (3): 367-77. -
Eleftheriou, E.P. et al. (2016) Hexavalent chromium-induced differential disruption of cortical microtubules in some Fabaceae species is correlated with acetylation of α-tubulin.
Protoplasma. 253 (2): 531-42. -
Okamoto, M. et al. (2016) Fyn Accelerates M Phase Progression by Promoting the Assembly of Mitotic Spindle Microtubules.
J Cell Biochem. 117 (4): 894-903. -
Sullivan, A.E. et al. (2016) MAGED1 is a novel regulator of a select subset of bHLH PAS transcription factors.
FEBS J. 283 (18): 3488-502. -
Livanos P et al. (2016) Deliberate ROS production and auxin synergistically trigger the asymmetrical division generating the subsidiary cells in Zea mays stomatal complexes.
Protoplasma. 253 (4): 1081-99. -
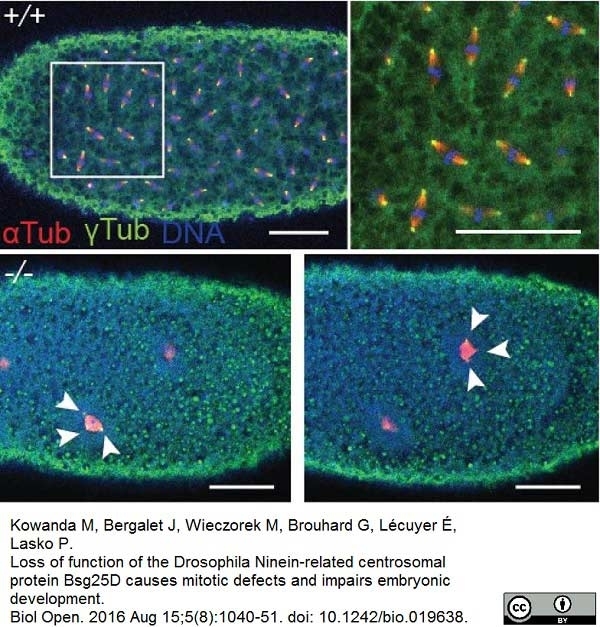
Kowanda, M. et al. (2016) Loss of function of the Drosophila Ninein-related centrosomal protein Bsg25D causes mitotic defects and impairs embryonic development.
Biol Open. 5 (8): 1040-51. -
Diao, L.T. et al. (2017) Delineation of the role of chromatin assembly and the Rtt101Mms1 E3 ubiquitin ligase in DNA damage checkpoint recovery in budding yeast.
PLoS One. 12 (7): e0180556. -
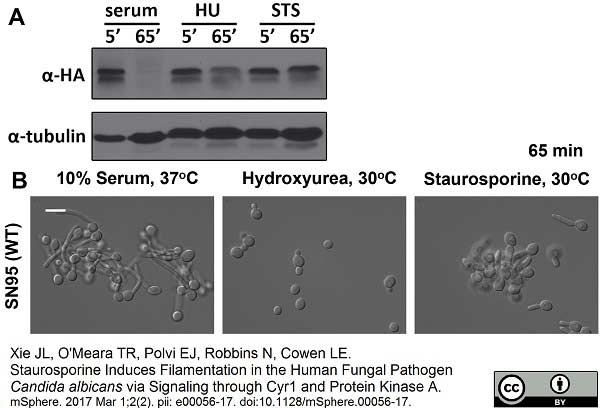
Xie, J.L. et al. (2017) Staurosporine Induces Filamentation in the Human Fungal Pathogen Candida albicans via Signaling through Cyr1 and Protein Kinase A.
mSphere. 2 (2): Mar 1;2(2). pii: e00056-17. eCollection 2017 Mar-Apr. -
Panteris, E. et al. (2018) Cortical microtubule orientation in Arabidopsis thaliana root meristematic zone depends on cell division and requires severing by katanin.
J Biol Res (Thessalon). 25: 12. -
Játiva, S. et al. (2019) Cdc14 activation requires coordinated Cdk1-dependent phosphorylation of Net1 and PP2A-Cdc55 at anaphase onset.
Cell Mol Life Sci. 76 (18): 3601-20. -
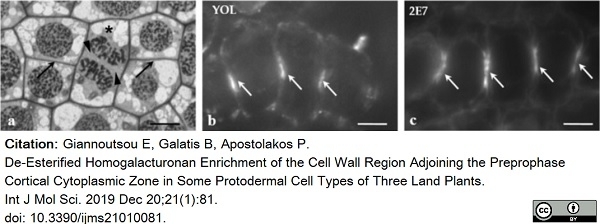
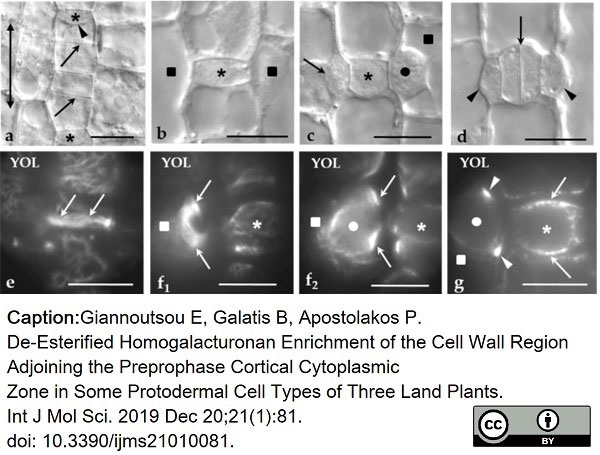
Giannoutsou, E. et al. (2019) De-Esterified Homogalacturonan Enrichment of the Cell Wall Region Adjoining the Preprophase Cortical Cytoplasmic Zone in Some Protodermal Cell Types of Three Land Plants.
Int J Mol Sci. 21(1):81. -
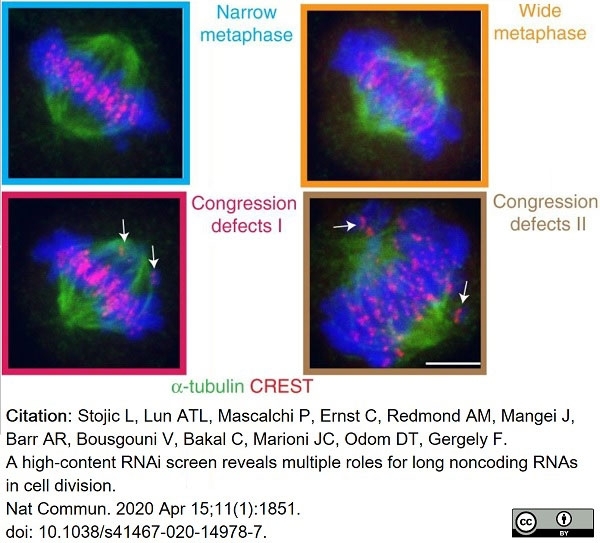
Stojic, L. et al. (2020) A high-content RNAi screen reveals multiple roles for long noncoding RNAs in cell division.
Nat Commun. 11 (1): 1851. -
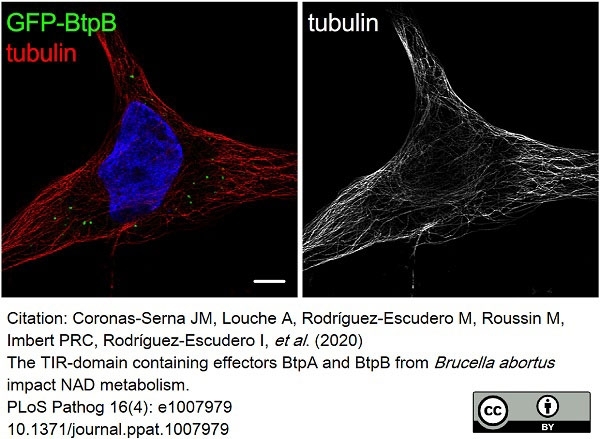
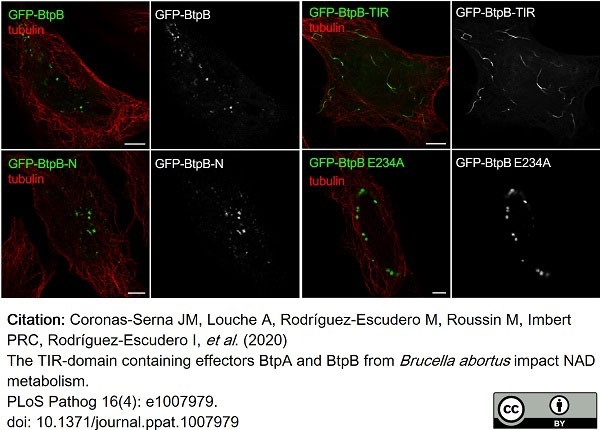
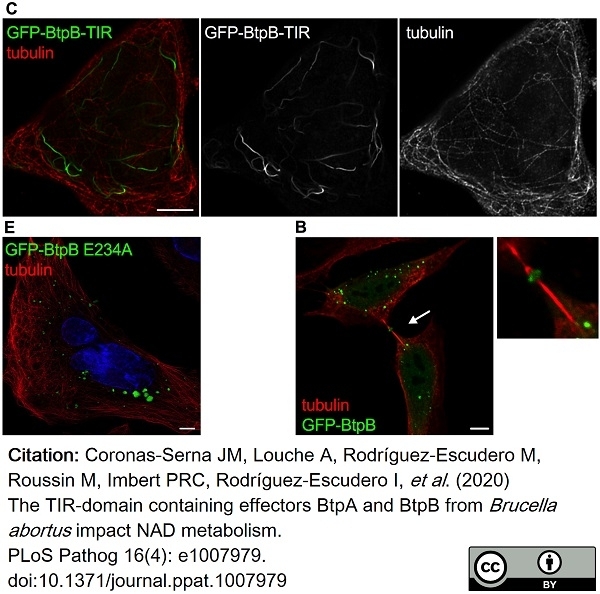
Coronas-Serna, J.M. et al. (2020) The TIR-domain containing effectors BtpA and BtpB from Brucella abortus impact NAD metabolism.
PLoS Pathog. 16 (4): e1007979. -
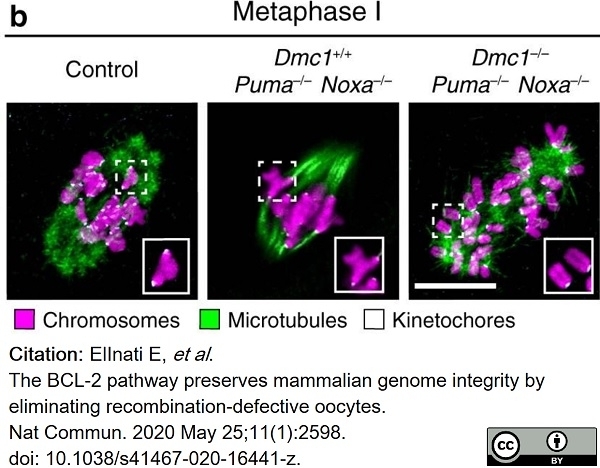
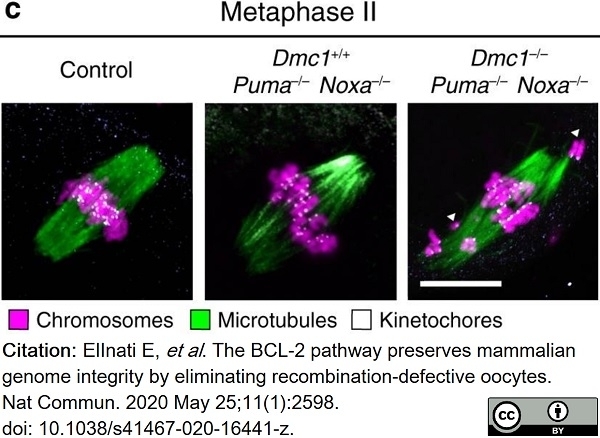
ElInati, E. et al. (2020) The BCL-2 pathway preserves mammalian genome integrity by eliminating recombination-defective oocytes.
Nat Commun. 11 (1): 2598. -
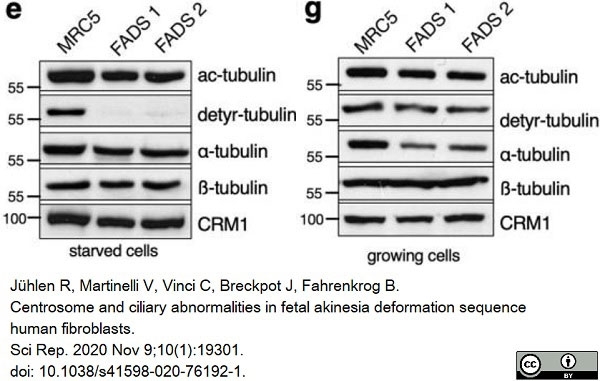
Jühlen, R. et al. (2020) Centrosome and ciliary abnormalities in fetal akinesia deformation sequence human fibroblasts.
Sci Rep. 10 (1): 19301. -
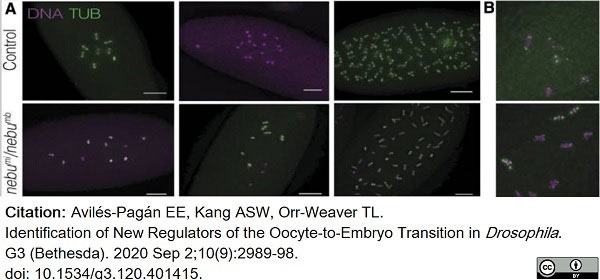
Avilés-Pagán, E.E. et al. (2020) Identification of New Regulators of the Oocyte-to-Embryo Transition in Drosophila.
G3 (Bethesda). 10 (9): 2989-2998. -
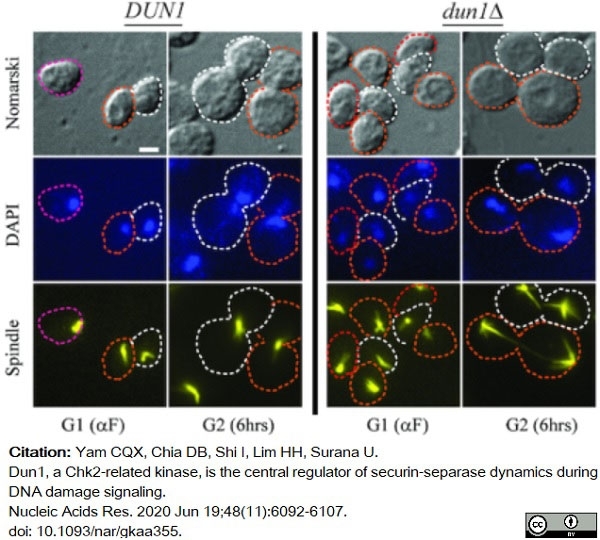
Yam, C.Q.X. et al. (2020) Dun1, a Chk2-related kinase, is the central regulator of securin-separase dynamics during DNA damage signaling.
Nucleic Acids Res. 48 (11): 6092-107. -
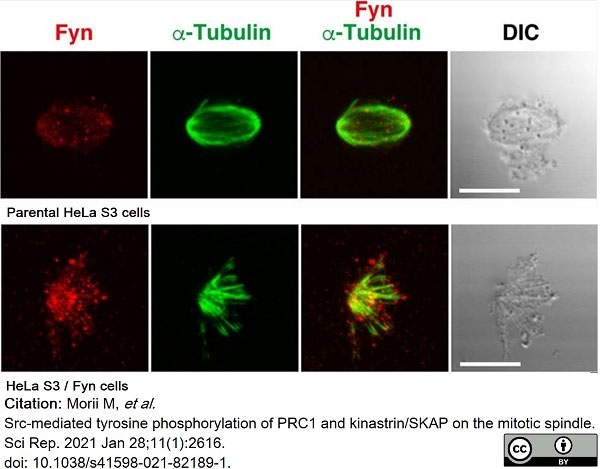
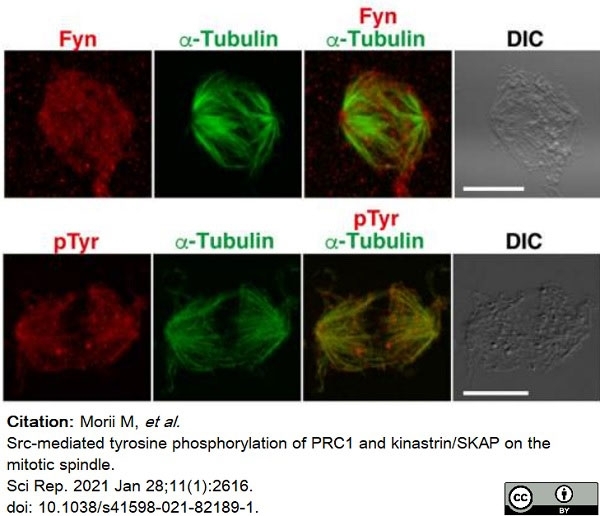
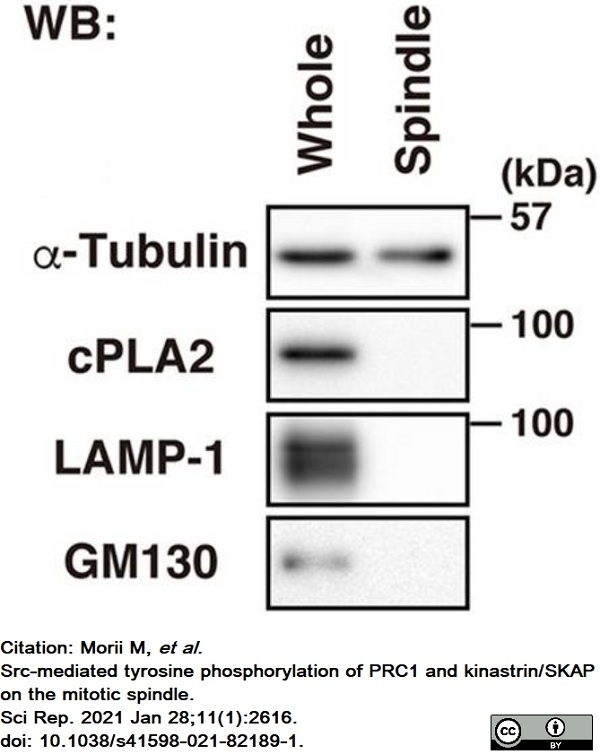
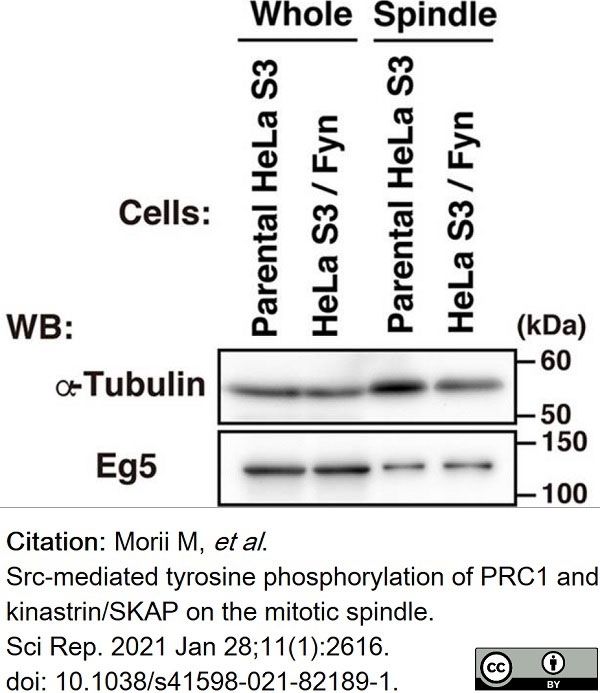
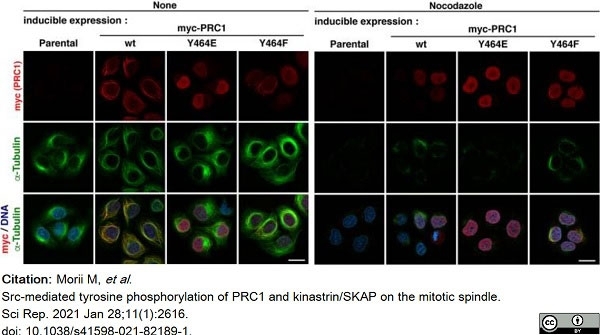
Morii, M. et al. (2021) Src-mediated tyrosine phosphorylation of PRC1 and kinastrin/SKAP on the mitotic spindle.
Sci Rep. 11 (1): 2616. -
Cavazza, T. et al. (2021) Parental genome unification is highly error-prone in mammalian embryos.
Cell. 184 (11): 2860-2877.e22. -
Giourieva, V. & Panteris, E. (2021) Inhibition of cell expansion enhances cortical microtubule stability in the root apex of Arabidopsis thaliana..
J Biol Res (Thessalon). 28 (1): 13. -
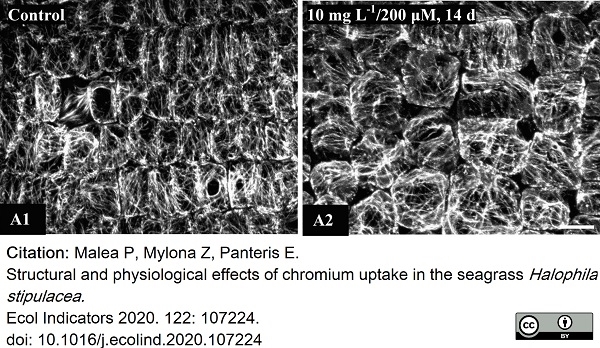
Malea, P. et al. (2021) Structural and physiological effects of chromium uptake in the seagrass Halophila stipulacea..
Ecological Indicators. 122: 107224. -
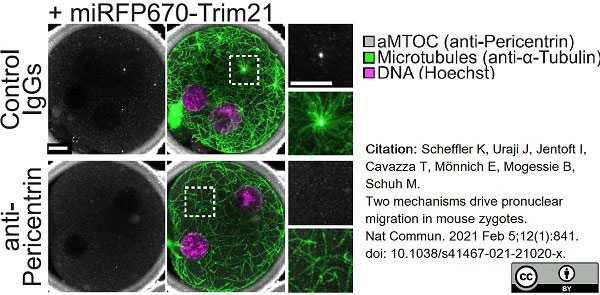
Scheffler, K. et al. (2021) Two mechanisms drive pronuclear migration in mouse zygotes.
Nat Commun. 12 (1): 841. -
Benoit, L.B. et al. (2023) RBP Image Database: A resource for the systematic characterization of the subcellular distribution properties of human RNA binding proteins.
Nucleic Acids Res. 51 (D1): D1549-D1557. -
Mourer, T. et al. (2023) The Pga59 cell wall protein is an amyloid forming protein involved in adhesion and biofilm establishment in the pathogenic yeast Candida albicans.
NPJ Biofilms Microbiomes. 9 (1): 6. -
Wu, S. et al. (2023) Apical-basal polarity precisely determines intestinal stem cell number by regulating Prospero threshold.
Cell Rep. 42 (2): 112093. -
Choudhary, R. et al. (2023) Sen1 and Rrm3 ensure permissive topological conditions for replication termination.
Cell Rep. 42 (7): 112747. -
Rojas, J. et al. (2023) Spo13/MEIKIN ensures a Two-Division meiosis by preventing the activation of APC/C(Ama1) at meiosis I.
EMBO J. 42 (20): e114288. -
Ota, S. et al. (2023) Distinct effects of heat shock temperatures on mitotic progression by influencing the spindle assembly checkpoint
Exp Cell Res. 429 (2): 113672. -
Gililand, W.D. et al. (2024) A Cytological F1 RNAi Screen for Defects in Drosophila melanogaster Female Meiosis
bioRχiv. 15 Jan [Epub ahead of print]. -
Grigaitis, R. et al. (2020) Phosphorylation of the RecQ Helicase Sgs1/BLM Controls Its DNA Unwinding Activity during Meiosis and Mitosis.
Dev Cell. 53 (6): 706-723.e5. -
Pappas, D. et al. (2020) The effects of microcystin-LR in Oryza sativa root cells: F-actin as a new target of cyanobacterial toxicity.
Plant Biol (Stuttg). 22 (5): 839-49. -
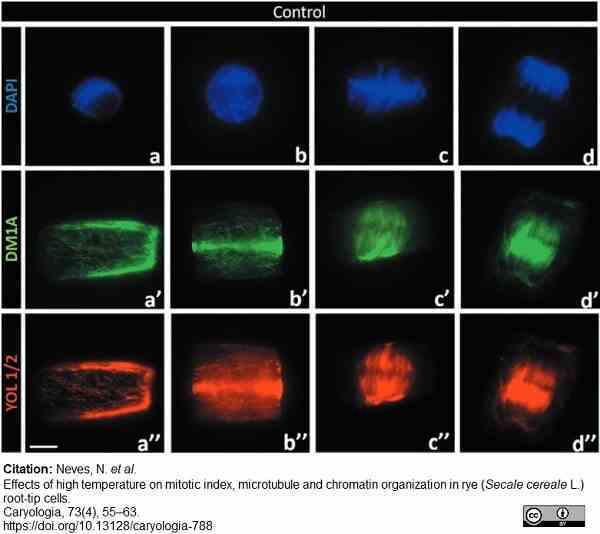
Neves, V. et al. (2020) Effects of high temperature on mitotic index, microtubule and chromatin organization in rye (Secale cereale L.) root-tip cells
Caryologia 73 (4): 55-63. -
Koutalianou, M. et al. (2022) In situ experiments on the effect of low pH on the ultrastructure of the seagrasses Cymodocea nodosa and Posidonia oceanica.
Mediterranean Marine Science. 23 (1), 30-45.
- RRID
- AB_325005
MCA78G
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Yeast ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up