Tubulin Alpha antibody | YL1/2


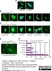


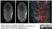



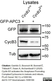
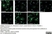
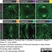


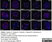
















Rat anti Tubulin Alpha
- Product Type
- Monoclonal Antibody
- Clone
- YL1/2
- Isotype
- IgG2a
- Specificity
- Tubulin Alpha
| Rat anti tubulin alpha antibody, clone YL1/2 recognizes the alpha subunit of tubulin, specifically binding tyrosylated Tubulin (Tyr-Tubulin) (Wehland et al. 1983). The epitope recognized by this antibody has been extensively studied and would appear to be a linear sequence requiring an aromatic residue at the C terminus, with the two adjacent amino acids being negatively charged (represented by Glu-Glu-Tyr in Tyr-Tubulin). The antibody has been used in epitope tagging procedures to detect proteins tagged with a C-terminal Gly-Gly-Phe epitope. These sequence requirements have been reported to result in some cross-reactivity with other proteins in certain circumstances, including E. coli rec A and oxidized actin (Burns 1987). Rat anti tubulin alpha antibody, clone YL1/2 is routinely tested in ELISA on tubulin. |
- Target Species
- Yeast
- Species Cross-Reactivity
-
Target Species Cross Reactivity Ashbya Birds Expected from Sequence Echinoderm Expected from Sequence Human Mouse Dog Rat Pig Drosophila Plants Expected from Sequence Amphibia Expected from Sequence Saccharomyces Pleurobrachia Caenorhabditis Dictyostelium discoideum Xenopus Pig-tailed macaque Clytia sp. Arabidopsis Strongylocentrotus purpuratus Dendraster excentricus Trypanosoma brucei Potorous tridactylis Bovine Nephrotoma suturalis Hemicentrotus pulcherrimus Potato Bombyx mori Rhodnius prolixus Beroe abyssicola Candida sp. - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant.
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
0.09% Sodium Azide - Carrier Free
- Yes
- Immunogen
- Yeast tubulin.
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunized LOU rats were fused with cells of the Y3.Ag.1.2.3 rat myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA | 1/100 | 1/1000 | |
| Immunofluorescence | |||
| Immunohistology - Frozen | |||
| Immunoprecipitation | |||
| Radioimmunoassays | |||
| Western Blotting | 1/200 | 1/2000 |
- Western Blotting
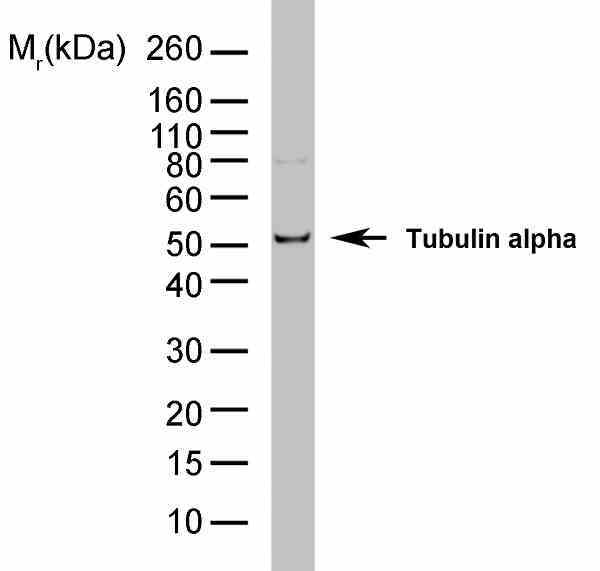
- Rat anti tubulin alpha antibody, clone YL1/2 recognizes a band of ~55 kDa in cell lysate from a wide range of species. Rat anti tubulin alpha antibody, clone YL1/2 is suitable for use as a western blotting loading control.
References for Tubulin Alpha antibody
-
Kilmartin, J.V. et al. (1982) Rat monoclonal anti tubulin antibodies derived by using a new nonsecreting rat cell line.
J Cell Biol. 93 (3): 576-82. -
Wehland, J. et al. (1983) A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. I. Biochemical characterization, effects on microtubule polymerization in vitro, and microtubule polymerization and organization in vivo.
J Cell Biol. 97 (5 Pt 1): 1467-75. -
Wehland, J. et al. (1984) Amino acid sequence requirements in the epitope recognized by the alpha-tubulin-specific rat monoclonal antibody YL 1/2.
EMBO J. 3 (6): 1295-300. -
Burns, R. (1987) Cytoskeleton. Tubulin's terminal tyrosine.
Nature. 327 (6118): 103-4. -
Skinner, R.H. et al. (1991) Use of the Glu-Glu-Phe C-terminal epitope for rapid purification of the catalytic domain of normal and mutant ras GTPase-activating proteins.
J Biol Chem. 266 (22): 14163-6. -
Berrueta, L. et al. (1998) The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules.
Proc Natl Acad Sci U S A. 95: 10596-601. -
Gordon-Weeks, R. et al. (2003) Restricted spatial expression of a high-affinity phosphate transporter in potato roots.
J Cell Sci.116: 3135-44. -
Ligon, L.A. et al. (2003) The microtubule plus-end proteins EB1 and dynactin have differential effects on microtubule polymerization.
Mol Biol Cell. 14: 1405-17.
View The Latest Product References
-
Müller, S. et al. (2004) The plant microtubule-associated protein AtMAP65-3/PLE is essential for cytokinetic phragmoplast function.
Curr Biol. 14: 412-7. -
Dorer, M.S. et al. (2006) RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics.
PLoS Pathog. 2(4): e34. -
Groeger, G. et al. (2007) Co-operative Cdc42 and Rho signalling mediates ephrinB-triggered endothelial cell retraction.
Biochem J. 404: 23-9. -
Machado, E. et al. (2007) Prostaglandin signaling and ovarian follicle development in the silkmoth, Bombyx mori.
Insect Biochem Mol Biol. 37: 876-85. -
Brunk, K. et al. (2007) Microcephalin coordinates mitosis in the syncytial Drosophila embryo.
J Cell Sci. 120: 3578-88. -
Smertenko, A.P. et al. (2008) The C-terminal variable region specifies the dynamic properties of Arabidopsis microtubule-associated protein MAP65 isotypes.
Plant Cell. 20: 3346-58. -
Tinkle, C.L. et al. (2008) New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity.
Proc Natl Acad Sci U S A. 105: 15405-10. -
Hartl, T.A. et al. (2008) Condensin II resolves chromosomal associations to enable anaphase I segregation in Drosophila male meiosis.
PLoS Genet. 4(10): e1000228. -
Jager, M. et al. (2008) Insights into the early evolution of SOX genes from expression analyses in a ctenophore.
J Exp Zool B Mol Dev Evol. 310: 650-67. -
von Dassow, G. et al. (2009) Action at a distance during cytokinesis.
J Cell Biol. 187: 831-45. -
Vafopoulou, X. (2009) Ecdysteroid receptor (EcR) is associated with microtubules and with mitochondria in the cytoplasm of prothoracic gland cells of Rhodnius prolixus (Hemiptera).
Arch Insect Biochem Physiol. 72: 249-62. -
Dupin, I. et al. (2009) Classical cadherins control nucleus and centrosome position and cell polarity.
J Cell Biol. 185: 779-86. -
Heaslip, A.T. et al. (2009) TgICMAP1 is a novel microtubule binding protein in Toxoplasma gondii.
PLoS One. 4: e7406. -
Towers, E. et al (2009) The proapoptotic dp5 gene is a direct target of the MLK-JNK-c-Jun pathway in sympathetic neurons.
Nucleic Acids Res. 37: 3044-60. -
Li, Y. et al. (2010) The type II Arabidopsis formin14 interacts with microtubules and microfilaments to regulate cell division.
Plant Cell. 22: 2710-26. -
Liu, D. et al. (2010) Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase.
J Cell Biol. 188: 809-20. -
Omri, S. et al. (2010) The outer limiting membrane (OLM) revisited: clinical implications.
Clin Ophthalmol. 4: 183-95. -
Wallace, S.W. et al. (2010) Cdc42 regulates apical junction formation in human bronchial epithelial cells through PAK4 and Par6B.
Mol Biol Cell. 21 (17): 2996-3006. -
Abe, Y. et al. (2010) A single starfish Aurora kinase performs the combined functions of Aurora-A and Aurora-B in human cells.
J Cell Sci. 123: 3978-88. -
Bruce, E.A. et al. (2010) The Rab11 pathway is required for influenza A virus budding and filament formation.
J Virol. 84: 5848-59. -
Zenner, H.L. et al. (2011) Analysis of Rab GTPase-Activating Proteins Indicates that Rab1a/b and Rab43 Are Important for Herpes Simplex Virus 1 Secondary Envelopment.
J Virol. 85: 8012-21. -
Wise, H.M. et al. (2011) Overlapping signals for translational regulation and packaging of influenza A virus segment 2.
Nucleic Acids Res. 39: 7775-90. -
Cheishvili, D. et al. (2011) Involvement in Cytoskeleton Regulation and Implication for Familial Dysautonomia.
Hum Mol Genet. 20: 1585-94. -
Wise, H.M. et al. (2011) Overlapping signals for translational regulation and packaging of influenza A virus segment 2.
Nucleic Acids Res. 39 (17): 7775-90. -
Stadler, L.K. et al. (2011) Structure-function studies of an engineered scaffold protein derived from Stefin A. II: Development and applications of the SQT variant.
Protein Eng Des Sel. 24 (9): 751-63. -
Morishita, D. et al. (2011) Cell-permeable carboxyl-terminal p27(Kip1) peptide exhibits anti-tumor activity by inhibiting Pim-1 kinase.
J Biol Chem. 286: 2681-8. -
Timm, T. et al. (2011) Microtubule affinity regulating kinase (MARK) activity in living neurons examined by a genetically encoded FRET/FLIM based biosensor: Inhibitors with therapeutic potential.
J Biol Chem. 286: 41711-22. -
Wise, H.M. et al. (2012) Identification of a novel splice variant form of the influenza A virus M2 ion channel with an antigenically distinct ectodomain.
PLoS Pathog. 8(11): e1002998. -
Vafopoulou, X. & Steel, C.G. (2012) Cytoplasmic travels of the ecdysteroid receptor in target cells: pathways for both genomic and non-genomic actions.
Front Endocrinol (Lausanne). 3: 43. -
Dayraud, C. et al. (2012) Independent specialisation of myosin II paralogues in muscle vs. non-muscle functions during early animal evolution: a ctenophore perspective.
BMC Evol Biol. 12: 107. -
Courtois, A. et al. (2012) The transition from meiotic to mitotic spindle assembly is gradual during early mammalian development.
J Cell Biol. 198: 357-70. -
Virágh, E. et al. (2012) Specific Cooperation Between Imp-α2 and Imp-β/Ketel in Spindle Assembly During Drosophila Early Nuclear Divisions.
G3 (Bethesda). 2 (1): 1-14. -
Meseroll, R.A. et al. (2012) Septin ring size scaling and dynamics require the coiled-coil region of Shs1p.
Mol Biol Cell. 23: 3391-406. -
Bodor, D.L. et al. (2013) Assembly in G1 phase and long-term stability are unique intrinsic features of CENP-A nucleosomes.
Mol Biol Cell. 24: 923-32. -
Feau, S. et al. (2013) SLAT Regulates CD8+ T Cell Clonal Expansion in a Cdc42- and NFAT1-Dependent Manner.
J Immunol. 190: 174-83. -
De Faveri, L.E. et al. (2013) Putative tumour suppressor gene necdin is hypermethylated and mutated in human cancer.
Br J Cancer. 108: 1368-77. -
Liz, M.A. et al. (2014) Neuronal deletion of GSK3β increases microtubule speed in the growth cone and enhances axon regeneration via CRMP-2 and independently of MAP1B and CLASP2.
BMC Biol. 12: 47. -
Levy, G.V. et al. (2015) Depletion of the SR-Related Protein TbRRM1 Leads to Cell Cycle Arrest and Apoptosis-Like Death in Trypanosoma brucei.
PLoS One. 10 (8): e0136070. -
Nunan, R. et al. (2015) Ephrin-Bs Drive Junctional Downregulation and Actin Stress Fiber Disassembly to Enable Wound Re-epithelialization.
Cell Rep. 13 (7): 1380-95. -
Koparir, A. et al. (2015) Novel POC1A mutation in primordial dwarfism reveals new insights for centriole biogenesis.
Hum Mol Genet. 24 (19): 5378-87. -
Gaudet, A.D. et al. (2015) Galectin-1 in injured rat spinal cord: implications for macrophage phagocytosis and neural repair.
Mol Cell Neurosci. 64: 84-94. -
Jonasson, E.M. et al. (2016) Zds1/Zds2-PP2ACdc55 complex specifies signaling output from Rho1 GTPase.
J Cell Biol. 212 (1): 51-61. -
Gholkar, A.A. et al. (2016) Fatostatin Inhibits Cancer Cell Proliferation by Affecting Mitotic Microtubule Spindle Assembly and Cell Division.
J Biol Chem. 291 (33): 17001-8. -
Kono, K. et al. (2016) Plasma membrane/cell wall perturbation activates a novel cell cycle checkpoint during G1 in Saccharomyces cerevisiae.
Proc Natl Acad Sci U S A. 113 (25): 6910-5. -
Schlicher, L. et al. (2016) SPATA2 promotes CYLD activity and regulates TNF-induced NF-κB signaling and cell death.
EMBO Rep. 17 (10): 1485-97. -
Vargas, P. et al. (2016) Innate control of actin nucleation determines two distinct migration behaviours in dendritic cells.
Nat Cell Biol. 18 (1): 43-53. -
Turnbull, M.L. et al. (2016) Role of the B Allele of Influenza A Virus Segment 8 in Setting Mammalian Host Range and Pathogenicity.
J Virol. 90 (20): 9263-84. -
Vafopoulou, X. & Steel, C.G.H. (2016) Mitochondria and the insect steroid hormone receptor (EcR): A complex relationship.
Gen Comp Endocrinol. 237: 68-77. -
Zasadil, L.M. et al. (2016) High rates of chromosome missegregation suppress tumor progression but do not inhibit tumor initiation.
Mol Biol Cell. 27 (13): 1981-9. -
Iwasaki, D. et al. (2016) The MRX Complex Ensures NHEJ Fidelity through Multiple Pathways Including Xrs2-FHA-Dependent Tel1 Activation.
PLoS Genet. 12 (3): e1005942. -
Kerr, G.W. et al. (2016) PP2A(Cdc55)'s role in reductional chromosome segregation during achiasmate meiosis in budding yeast is independent of its FEAR function.
Sci Rep. 6: 30397. -
Takáč, T. et al. (2017) Actin depolymerization-induced changes in proteome of Arabidopsis roots.
J Proteomics. 153: 89-99. -
Gao, L. et al. (2017) Afadin orients cell division to position the tubule lumen in developing renal tubules.
Development. 144 (19): 3511-20. -
Klinger, P. et al. (2017) PEDF Is Associated with the Termination of Chondrocyte Phenotype and Catabolism of Cartilage Tissue.
Biomed Res Int. 2017: 7183516. -
Inoue, D. et al. (2019) Actin filaments regulate microtubule growth at the centrosome.
EMBO J. 38(11): e99630. -
Norekian, T.P. & Moroz, L.L. (2019) Neural system and receptor diversity in the ctenophore Beroe abyssicola.
J Comp Neurol. 527 (12): 1986-2008. -
Sawicki, M.P. et al. (2019) Menin Associates With the Mitotic Spindle and Is Important for Cell Division.
Endocrinology. 160 (8): 1926-36. -
Norekian, T.P. & Moroz, L.L. (2019) Neuromuscular organization of the Ctenophore Pleurobrachia bachei.
J Comp Neurol. 527 (2): 406-36. -
Patteson, A.E. et al. (2019) Loss of Vimentin Enhances Cell Motility through Small Confining Spaces.
Small. 15 (50): e1903180. -
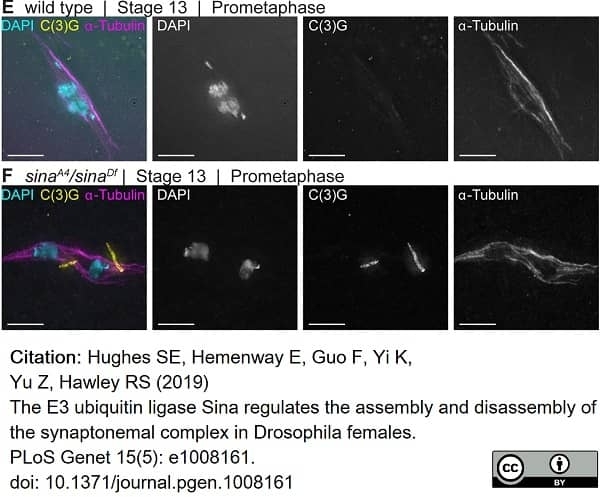
Hughes, S.E. et al. (2019) The E3 ubiquitin ligase Sina regulates the assembly and disassembly of the synaptonemal complex in Drosophila. females.
PLoS Genet. 15 (5): e1008161. -
Soday, L. et al. (2019) Quantitative Temporal Proteomic Analysis of Vaccinia Virus Infection Reveals Regulation of Histone Deacetylases by an Interferon Antagonist.
Cell Rep. 27 (6): 1920-1933.e7. -
Bernkopf, D.B. et al. (2019) An aggregon in conductin/axin2 regulates Wnt/β-catenin signaling and holds potential for cancer therapy.
Nat Commun. 10 (1): 4251. -
Lee, D.K. et al. (2019) Cdk5 regulates N-cadherin-dependent neuronal migration during cortical development.
Biochem Biophys Res Commun. 514 (3): 645-52. -
Montesinos, J.C. et al. (2020) Phytohormone cytokinin guides microtubule dynamics during cell progression from proliferative to differentiated stage.
EMBO J. 39 (17): e104238. -
Garrido, D. et al. (2020) Cyclin B3 activates the Anaphase-Promoting Complex/Cyclosome in meiosis and mitosis.
PLoS Genet. 16 (11): e1009184. -
Norekian, T.P. & Moroz, L.L. (2020) Comparative neuroanatomy of ctenophores: Neural and muscular systems in Euplokamis dunlapae and related species.
J Comp Neurol. 528 (3): 481-501. -
Norekian, T.P. & Moroz, L.L. (2020) Atlas of the neuromuscular system in the Trachymedusa aglantha digitale: Insights from the advanced hydrozoan.
J Comp Neurol. 528 (7): 1231-54. -
Gallaud, E. et al. (2020) Dynamic centriolar localization of Polo and Centrobin in early mitosis primes centrosome asymmetry.
PLoS Biol. 18 (8): e3000762. -
Norekian, T.P. & Moroz, L.L. (2021) Development of the nervous system in the early hatching larvae of the ctenophore Mnemiopsis leidyi..
J Morphol. 282 (10): 1466-1477. -
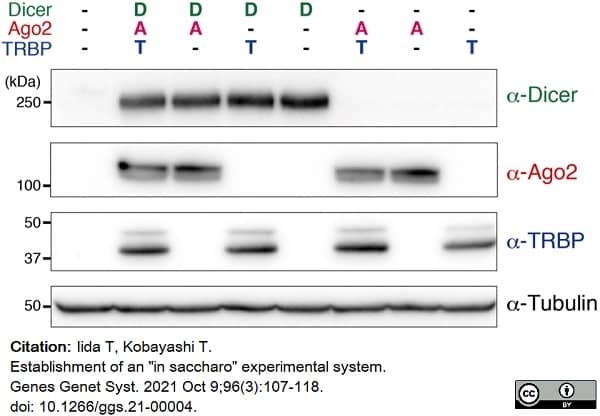
Iida, T. & Kobayashi, T. (2021) Establishment of an "in saccharo" experimental system.
Genes Genet Syst. 96 (3): 107-18. -
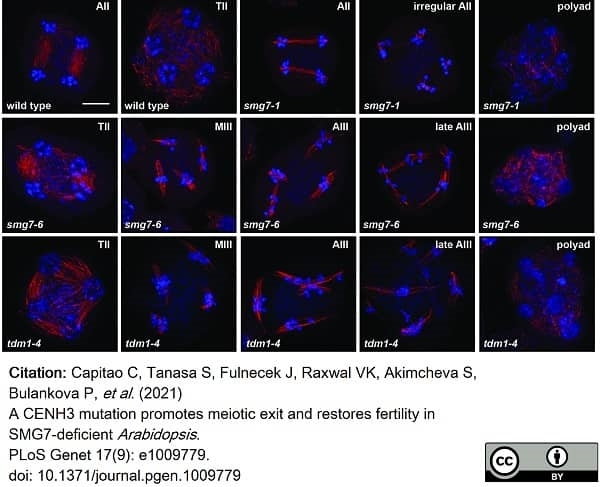
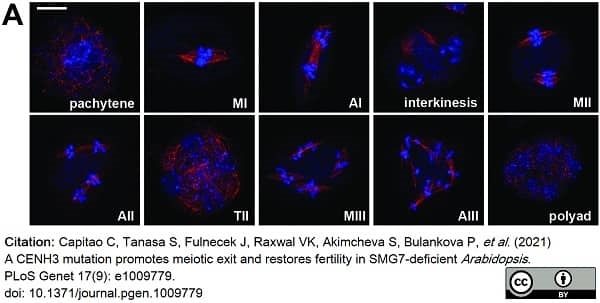
Capitao, C. et al. (2021) A CENH3 mutation promotes meiotic exit and restores fertility in SMG7-deficient Arabidopsis..
PLoS Genet. 17 (9): e1009779. -
Sprenger, M. et al. (2021) A TRP1-marker-based system for gene complementation, overexpression, reporter gene expression and gene modification in Candida glabrata.
FEMS Yeast Res.20(8):foaa066. -
Garcia, Y.A. et al. (2021) Mapping Proximity Associations of Core Spindle Assembly Checkpoint Proteins.
J Proteome Res. 20 (7): 3414-27. -
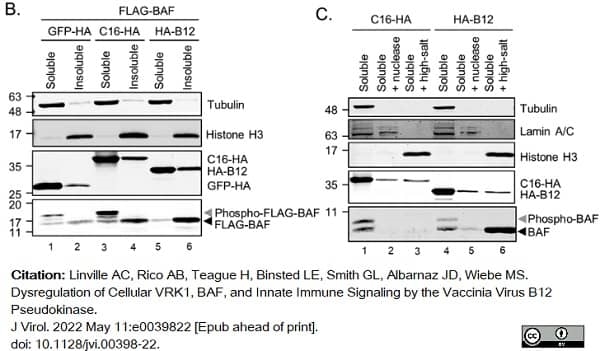
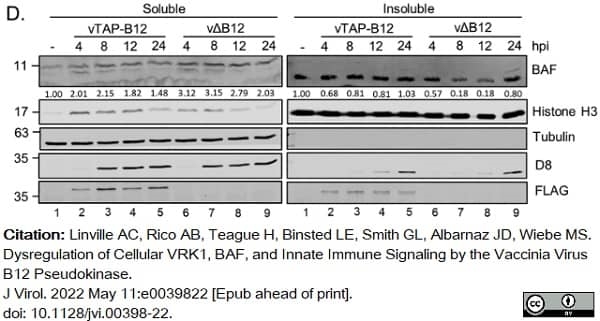
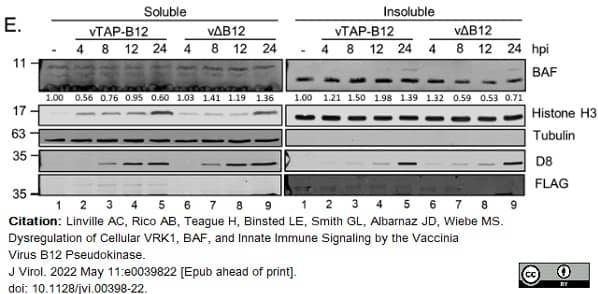
Linville, A.C. et al. (2022) Dysregulation of Cellular VRK1, BAF, and Innate Immune Signaling by the Vaccinia Virus B12 Pseudokinase.
J Virol. : e0039822. -
Miete, C. et al. (2022) Gαi2-induced conductin/axin2 condensates inhibit Wnt/β-catenin signaling and suppress cancer growth.
Nat Commun. 13 (1): 674. -
Carnesecchi, J. et al. (2022) The Hox transcription factor Ultrabithorax binds RNA and regulates co-transcriptional splicing through an interplay with RNA polymerase II.
Nucleic Acids Res. 50 (2): 763-783. -
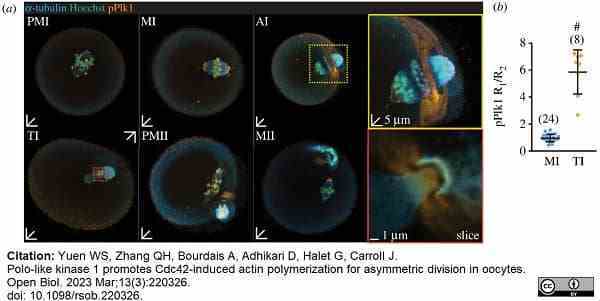
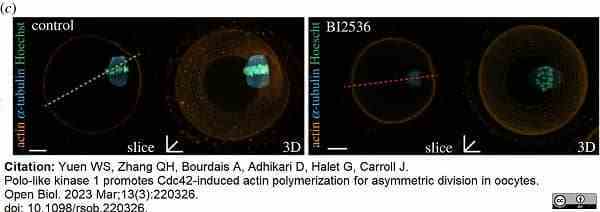
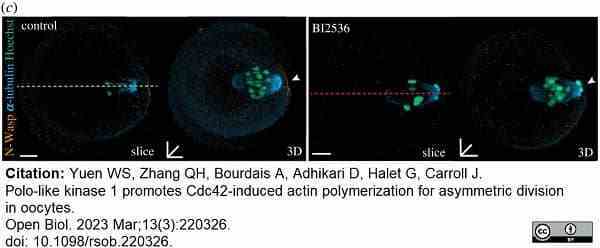
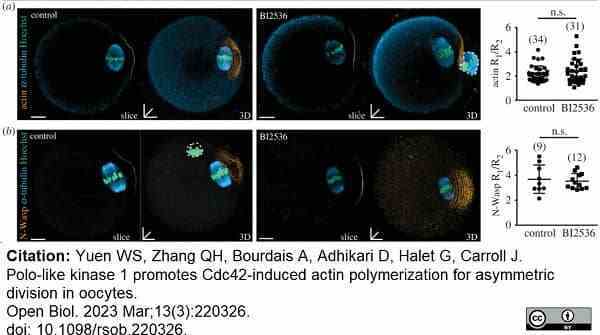
Yuen, S.W. et al. (2023) Polo-like kinase 1 promotes Cdc42-induced actin polymerization for asymmetric division in oocytes
Open Biology. 13 (3): 220326. -
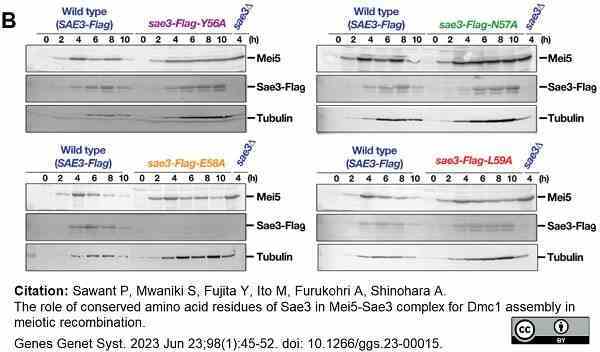
Sawant, P. et al. (2023) The role of conserved amino acid residues of Sae3 in Mei5-Sae3 complex for Dmc1 assembly in meiotic recombination.
Genes Genet Syst. 98 (1): 45-52. -
Yang, S. et al. (2023) Autoinhibitory mechanism controls binding of centrosomin motif 1 to γ-tubulin ring complex.
J Cell Biol. 222 (7): e202007101. -
Numata-Uematasu, Y. et al. (2023) In vitro myelination using explant culture of dorsal root ganglia: An efficient tool for analyzing peripheral nerve differentiation and disease modeling.
PLoS One. 18 (5): e0285897. -
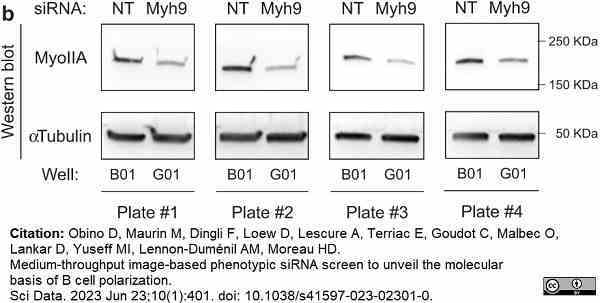
Obino, D. et al. (2023) Medium-throughput image-based phenotypic siRNA screen to unveil the molecular basis of B cell polarization.
Sci Data. 10 (1): 401. -
Caplan, T. et al. (2018) Functional Genomic Screening Reveals Core Modulators of Echinocandin Stress Responses in Candida albicans.
Cell Rep. 23 (8): 2292-2298. -
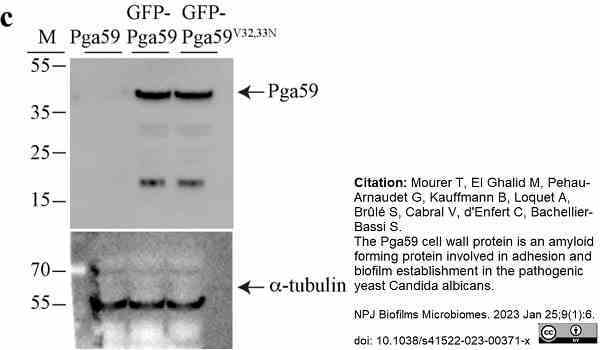
Mourer, T. et al. (2023) The Pga59 cell wall protein is an amyloid forming protein involved in adhesion and biofilm establishment in the pathogenic yeast Candida albicans.
NPJ Biofilms Microbiomes. 9 (1): 6. -
Bartels, C.B. et al. (2021) Short-term testosterone use in female mice does not impair fertilizability of eggs: implications for the fertility care of transgender males.
Hum Reprod. 36 (1): 189-98. -
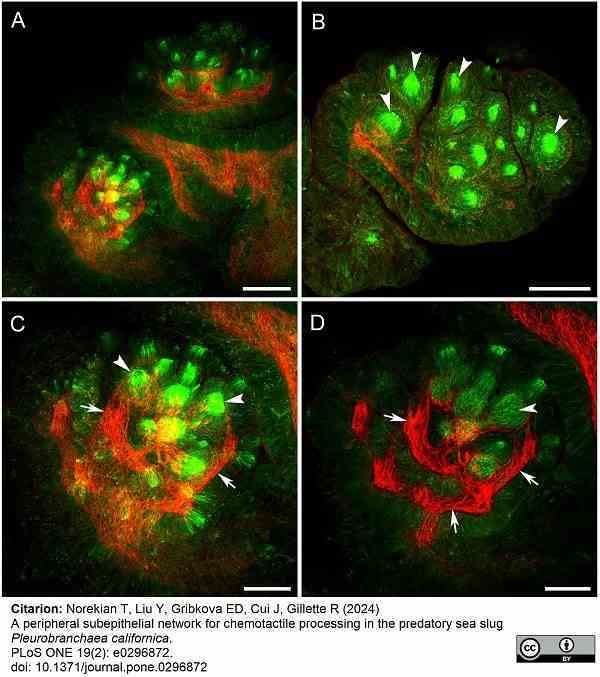
Norekian, T. et al. (2024) A peripheral subepithelial network for chemotactile processing in the predatory sea slug Pleurobranchaea californica.
PLoS One. 19 (2): e0296872. -
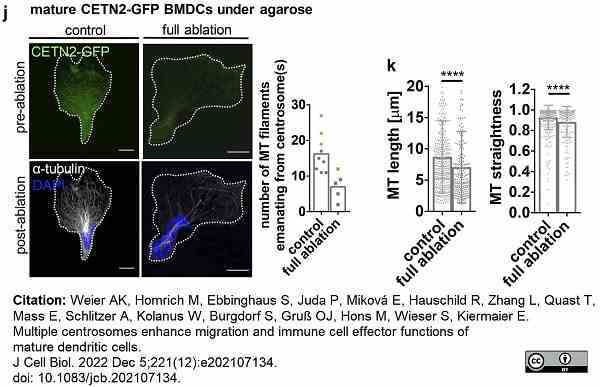
Weier, A.K. et al. (2022) Multiple centrosomes enhance migration and immune cell effector functions of mature dendritic cells.
J Cell Biol. 221 (12): e202107134. -
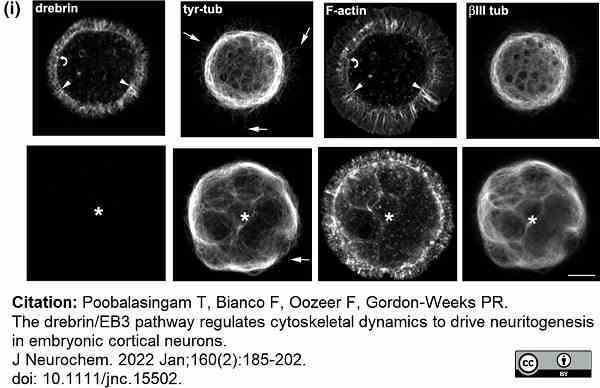
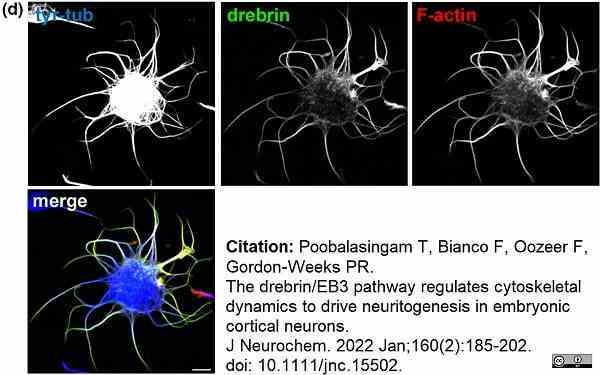
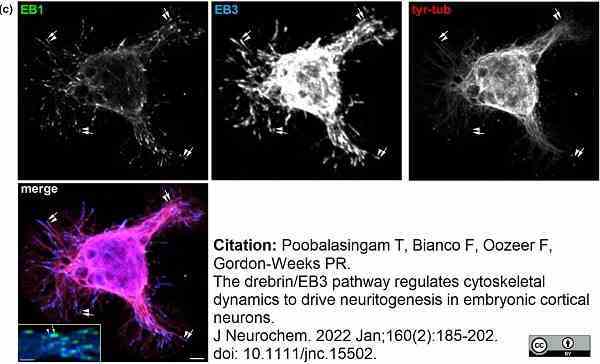
Poobalasingam, T. et al. (2022) The drebrin/EB3 pathway regulates cytoskeletal dynamics to drive neuritogenesis in embryonic cortical neurons.
J Neurochem. 160 (2): 185-202. -
Prasada Rao, H.BD. et al. (2021) Phosphorylation of luminal region of the SUN-domain protein Mps3 promotes nuclear envelope localization during meiosis.
Elife. 10: e63119.
- RRID
- AB_325003
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Yeast ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up