Substance P antibody | NC1/34




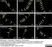


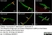
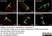













Rat anti Substance P
- Product Type
- Monoclonal Antibody
- Clone
- NC1/34
- Isotype
- IgG2a
- Specificity
- Substance P
| Rat anti substance P antibody, clone NC1/34 recognizes the COOH terminal region of substance P, an eleven amino acid polypeptide neurotransmitter derived by proteolytic cleavage of protachykinin-1. Substance P binds to and activates the neurokinin-1 receptor. Substance P is a highly conserved neurotransmitter expressed throughout the animal kingdom. It is highly expressed in the spinal cord dorsal horn and dorsal root ganglia, but not in the ventral horn (Ribeiro-da-Silva andT Hökfelt 2000). It is also expressed in non-neuronal cells including microglia and other immune cells. Substance P has a role in several physiologic activities such as pain transmission, the vomiting reflex, salivary secretion and smooth muscle contraction (Hökfelt 2004). Rat anti substance P antibody, clone NC1/34 demonstrates ~5% reactivity with eledoisin a neurotransmitter identified in the octopus Genus Eledone. It does not react with Leu or Met-enkephalin, somatostatin or beta-endorphin in tested applications. |
- Species Cross-Reactivity
-
Target Species Cross Reactivity Mammals Expected from Sequence Pig Pigeon Bichir Rabbit Human Bovine Guinea Pig Calanus sp. Crustacea Expected from Sequence Cancer sp. Panilurus sp. Homarus sp. Mudpuppy Lamprey Crayfish Zebrafish Bullfrog Amphibia Expected from Sequence Cockroach Sheep Cowbird Rat Spadefoot toad Chicken Chinchilla Camel Beaver Chinchilla - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Tissue Culture Supernatant - liquid
- Preservative Stabilisers
- 0.09% Sodium Azide (NaN3)
- Immunogen
- Substance P conjugated to bovine serum albumin.
- Fusion Partners
- Spleen cells from immunized Wistar rat were fused with cells of the NS1/1-Ag 4-1 myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Immunofluorescence | |||
| Immunohistology - Frozen | 1/100 | 1/200 | |
| Immunohistology - Paraffin 1 | 1/100 | 1/200 |
- 1This product does not require antigen retrieval prior to staining of formalin fixed, paraffin embedded sections.
References for Substance P antibody
-
Kozsurek, M. et al. (2009) Nonselective innervation of lamina I projection neurons by cocaine- and amphetamine-regulated transcript peptide (CART)-immunoreactive fibres in the rat spinal dorsal horn.
Eur J Neurosci. 29: 2375-87. -
Cuello, A.C. et al. (1980) Use of monoclonal antibodies in immunocytochemistry with special reference to the central nervous system.
Brain Res Bull. 5 (5): 575-87. -
Pioro, E.P. et al. (1985) Loss of substance P immunoreactivity in the nucleus of the spinal trigeminal tract after intradural tumour compression of the trigeminal nerve.
Neurosci Lett. 58: 7-12. -
Mai, J.K. et al. (1986) Substance P in the human brain.
Neuroscience. 17 (3): 709-39. -
Cuello, A.C. et al. (1979) Detection of substance P in the central nervous system by a monoclonal antibody.
Proc Natl Acad Sci U S A. 76 (7): 3532-6. -
Czujkowska, H. & Arciszewski, M.B. (2011) Expression of corticotropin releasing factor (CRF) in the enteric nervous system of the jejunum of sheep
Veterinarni medicina 56: 551-60. -
Kato, A. et al. (2012) Endocannabinoid-dependent plasticity at spinal nociceptor synapses.
J Physiol. 590 (Pt 19): 4717-33. -
Arciszewski, M.B. et al. (2009) Expression of vasoactive intestinal polypeptide, substance P and neuropeptide Y in jejunal enteric nerves is altered in rabbits suffering from long term Trichinella spiralis infection: an immunohistochemical study
Veterinarni Medicina, 54: 589–97
View The Latest Product References
-
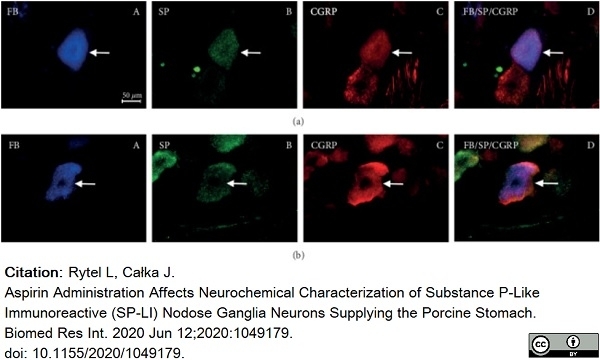
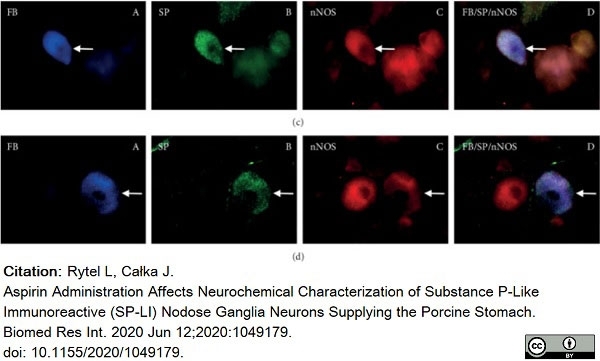
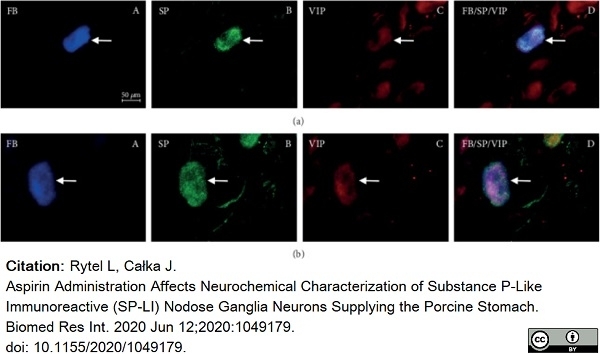
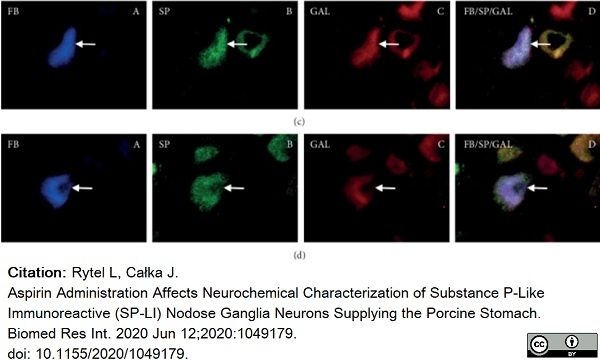
Bulc, M. et al. (2013) Immunohistochemical characterization of the porcine nodose ganglion.
Acta Histochem. 115 (5): 440-6. -
Boorsma, D.M. et al. (1982) Direct immunocytochemistry with a horseradish peroxidase-conjugated monoclonal antibody against substance P.
J Histochem Cytochem. 30: 1211-6. -
Pioro, E.P. et al. (1984) Loss of substance P and Enkephalin immunoreactivity in the human substantia nigra after striato-pallidal infarction
Brain Res. 292: 339-47. -
Mortimer, S.T. et al. (1990) Immunohistochemical identification of calcitonin gene-related peptide and substance P in nerves of the bovine parathyroid gland.
Cell Tissue Res. 261: 339-45. -
Galligan, J.J. et al. (1988) Changes in surviving nerve fibers associated with submucosal arteries following extrinsic denervation of the small intestine.
Cell Tissue Res. 253: 647-56. -
Horn, J.P. and Stofer, W.D. (1989) Preganglionic and sensory origins of calcitonin gene-related peptide-like and substance P-like immunoreactivities in bullfrog sympathetic ganglia.
J Neurosci. 9: 2543-61. -
Verhaert, P. & De Loof, A. (1985) Substance P-like immunoreactivity in the central nervous system of the blattarian insect Periplaneta americana L. revealed by a monoclonal antibody.
Histochemistry. 83: 501-7. -
Polgár, E. et al. (2007) A population of large neurons in laminae III and IV of the rat spinal cord that have long dorsal dendrites and lack the neurokinin 1 receptor.
Eur J Neurosci. 26: 1587-98. -
Nowicki, M. et al. (2007) Peptidergic and nitrergic innervation of the pineal gland in the domestic pig: an immunohistochemical study.
Anat Histol Embryol. 36: 311-20. -
Nair-Roberts, R.G. et al. (2006) Distribution of substance P reveals a novel subdivision in the hippocampus of parasitic South American cowbirds.
J Comp Neurol. 496: 610-26. -
Song, Y. et al. (2013) Bilateral increase in expression and concentration of tachykinin in a unilateral rabbit muscle overuse model that leads to myositis.
BMC Musculoskelet Disord. 14: 134. -
Szczurkowski, A. et al. (2013) Morphology and immunohistochemical characteristics of the pterygopalatine ganglion in the chinchilla (Chinchilla laniger, Molina).
Pol J Vet Sci. 16 (2): 359-68. -
Pidsudko, Z. (2014) Immunohistochemical characteristics and distribution of sensory dorsal root Ganglia neurons supplying the urinary bladder in the male pig.
J Mol Neurosci. 52 (1): 71-81. -
Zacharko-Siembida, A. et al. (2014) Co-expression patterns of cocaine- and amphetamine-regulated transcript (CART) with neuropeptides in dorsal root ganglia of the pig.
Acta Histochem. 116 (2): 390-8. -
Dudek, A. et al. (2012) Immunohistochemical characterization of neurons in the vestibular ganglion (Scarpa's ganglion) of the pig.
Pol J Vet Sci.15: 499-507. -
Ibrahim, D. et al. (2015) Immunohistochemical studies for the neuronal elements in the vomeronasal organ of the one-humped camel.
J Vet Med Sci. 77 (2): 241-5. -
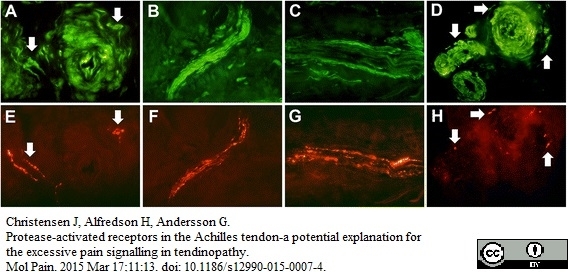
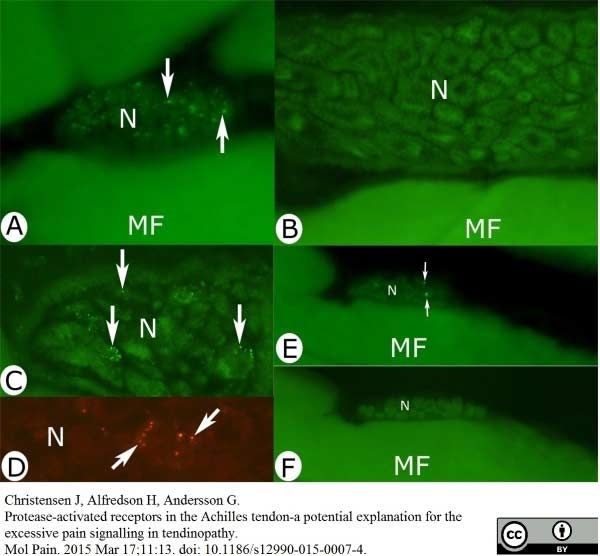
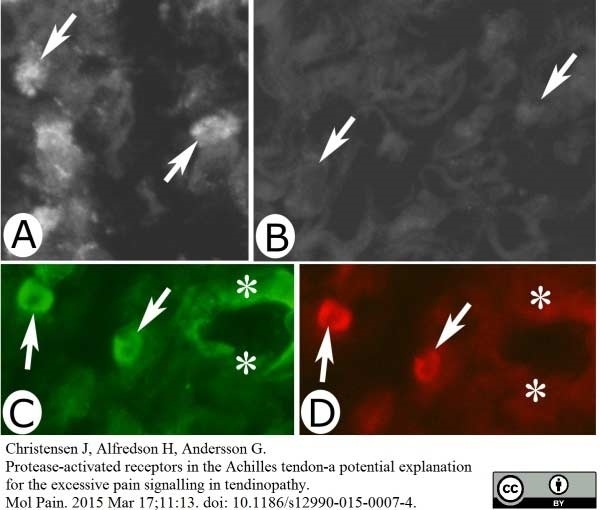
Christensen, J. et al. (2015) Protease-activated receptors in the Achilles tendon-a potential explanation for the excessive pain signalling in tendinopathy.
Mol Pain. 11: 13. -
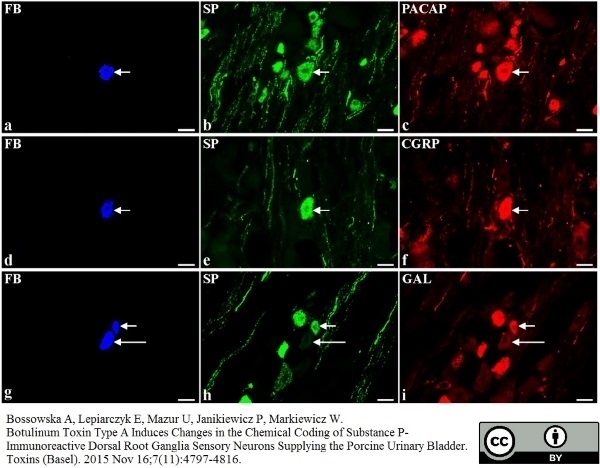
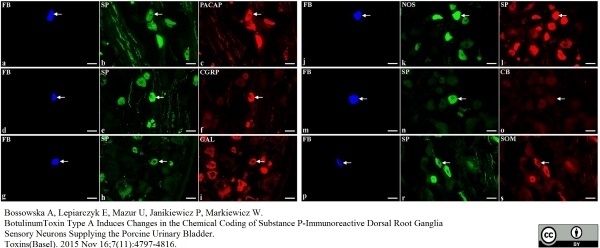
Bossowska, A. et al. (2015) Botulinum Toxin Type A Induces Changes in the Chemical Coding of Substance P-Immunoreactive Dorsal Root Ganglia Sensory Neurons Supplying the Porcine Urinary Bladder.
Toxins (Basel). 7 (11): 4797-816. -
Sienkiewicz, W. et al. (2015) Innervation of the chinchilla testis, epididymis, and vas deferens
Bull Vet Inst Pulawy. 59 (4): 547-56. -
Czujkowska, A. & Arciszewski, M.B. (2016) Galanin is Co-Expressed with Substance P, Calbindin and Corticotropin-Releasing Factor (CRF) in The Enteric Nervous System of the Wild Boar (Sus scrofa) Small Intestine.
Anat Histol Embryol. 45 (2): 115-23. -
Klimczuk, M. et al. (2016) Immunohistochemical characterisation of neurons in the mandibular ganglion and nerve fibres supplying the porcine mandibular gland.
Veterinarni Medicina. 61: 361-73. -
Gańko, M. & Całka, J. (2014) Localization and chemical coding of the dorsal motor vagal nucleus (DMX) neurons projecting to the porcine stomach prepyloric area in the physiological state and after stomach partial resection.
J Mol Neurosci. 52 (1): 90-100. -
Pidsudko, Z. et al. (2008) Distribution and chemical coding of intramural neurons in the porcine ileum during proliferative enteropathy.
J Comp Pathol. 138 (1): 23-31. -
Kaleczyc, J. et al. (2007) The distribution and chemical coding of intramural neurons supplying the porcine stomach - the study on normal pigs and on animals suffering from swine dysentery.
Anat Histol Embryol. 36 (3): 186-93. -
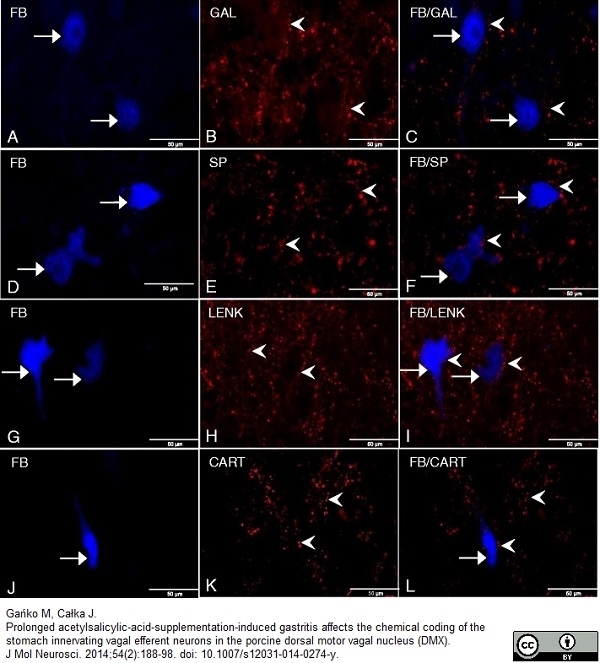
Gańko M & Całka J (2014) Prolonged acetylsalicylic-acid-supplementation-induced gastritis affects the chemical coding of the stomach innervating vagal efferent neurons in the porcine dorsal motor vagal nucleus (DMX).
J Mol Neurosci. 54 (2): 188-98. -
Dudek, A. et al. (2017) Chemical Coding of Sensory Neurons Supplying the Hip Joint Capsule in the Sheep.
Anat Histol Embryol. 46 (2): 121-31. -
Zacharko-Siembida, A. et al. (2017) An Immunohistochemical Study of Cocaine- and Amphetamine-Regulated Transcript (Cart) Expression in the Pterygopalatine Ganglion of the Pig
Acta Veterinaria. 67 (3): 397-408. -
Rytel, L. et al. (2018) Neurochemical characterization of intramural nerve fibres in the porcine oesophagus.
Anat Histol Embryol. 47 (6): 517-26. -
Zalecki, M. (2019) Gastric ulcer induced changes in substance P and Nk1, Nk2, Nk3 receptors expression in different stomach localizations with regard to intrinsic neuronal system.
Histochem Cell Biol. 151 (1): 29-42. -
Zalecki, M. et al. (2019) Enteric nervous system in the European beaver (Castor fiber) pylorus - an immunohistochemical study.
Pol J Vet Sci. 22 (1): 101-7. -
Sienkiewicz, W. et al. (2015) Caudal mesenteric ganglion in the sheep - macroanatomical and immunohistochemical study.
Pol J Vet Sci. 18 (2): 379-89. -
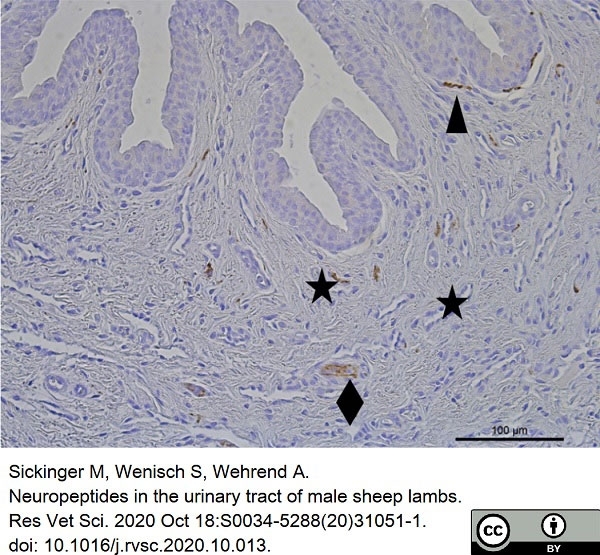
Sickinger, M. et al. (2020) Neuropeptides in the urinary tract of male sheep lambs.
Res Vet Sci. 133: 307-12. -
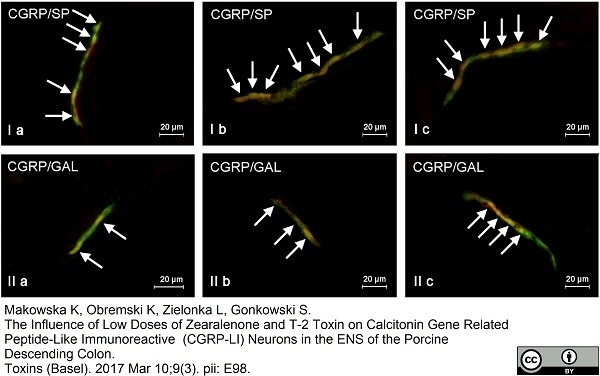
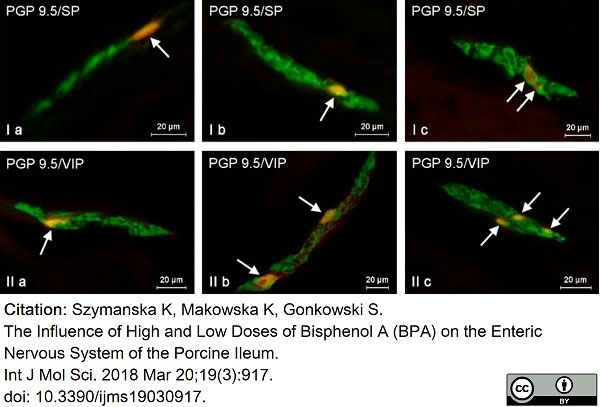
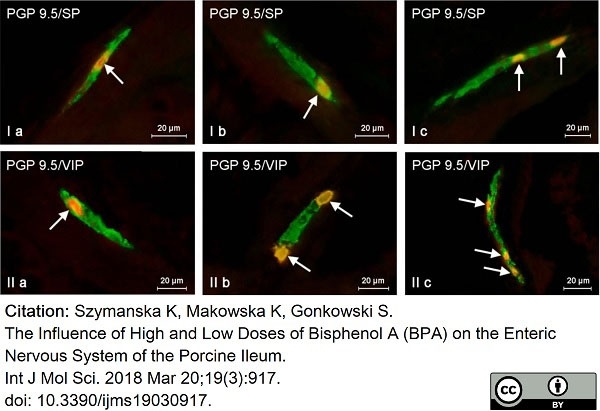
Szymanska, K. et al. (2018) The Influence of High and Low Doses of Bisphenol A (BPA) on the Enteric Nervous System of the Porcine Ileum.
Int J Mol Sci. 19(3 ): 917. -
Rytel, L. & Całka, J. (2020) Aspirin Administration Affects Neurochemical Characterization of Substance P-Like Immunoreactive (SP-LI) Nodose Ganglia Neurons Supplying the Porcine Stomach.
Biomed Res Int. 2020: 1049179. -
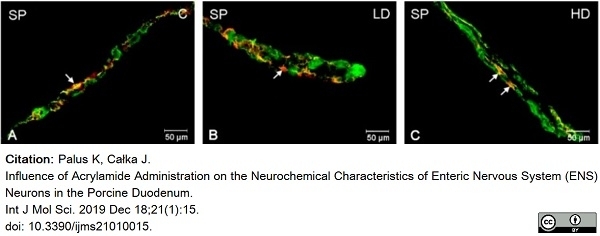
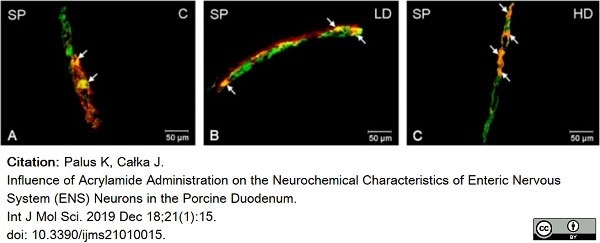
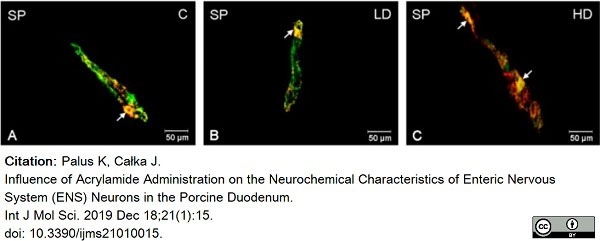
Palus, K. & Całka, J. (2019) Influence of Acrylamide Administration on the Neurochemical Characteristics of Enteric Nervous System (ENS) Neurons in the Porcine Duodenum.
Int J Mol Sci. 21 (1): 15. -
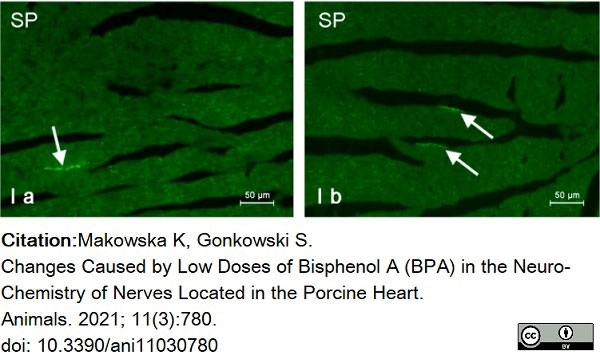
Makowska, K. & Gonkowski, S. (2021) Changes Caused by Low Doses of Bisphenol A (BPA) in the Neuro-Chemistry of Nerves Located in the Porcine Heart
Animals. 11 (3): 780. -
Bulc, M. et al. (2019) Endometritis affects chemical coding of the dorsal root ganglia neurons supplying uterus in the sexually mature gilts.
Res Vet Sci. 124: 417-25. -
Bulc, M. et al. (2018) Changes in Immunoreactivity of Sensory Substances within the Enteric Nervous System of the Porcine Stomach during Experimentally Induced Diabetes.
J Diabetes Res. 2018: 4735659. -
Palus, K. et al. (2019) The impact of low and high doses of acrylamide on the intramural neurons of the porcine ileum.
Food Chem Toxicol. 132: 110673. -
Meregalli, C. et al. (2021) Reversal of Bortezomib-Induced Neurotoxicity by Suvecaltamide, a Selective T-Type Ca-Channel Modulator, in Preclinical Models.
Cancers (Basel). 13 (19): 5013. -
Palus, K. et al. (2018) Changes in VIP-, SP- and CGRP- like immunoreactivity in intramural neurons within the pig stomach following supplementation with low and high doses of acrylamide.
Neurotoxicology. 69: 47-59. -
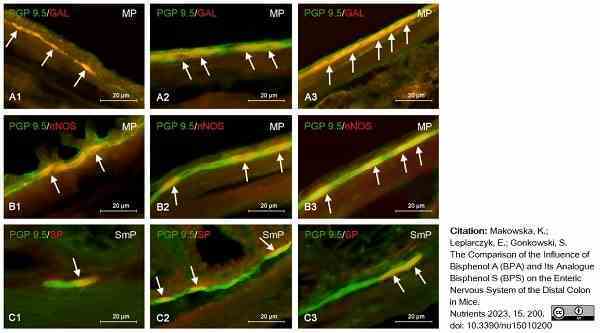
Makowska, K. et al. (2022) The Comparison of the Influence of Bisphenol A (BPA) and Its Analogue Bisphenol S (BPS) on the Enteric Nervous System of the Distal Colon in Mice
Nutrients. 15 (1): 200. -
Sienkiewicz, W. et al. (2023) Morphology and immunohistochemical characteristics of the otic ganglion in the chinchilla (Chinchilla laniger Molina).
Folia Histochem Cytobiol. 61 (1): 17-25. -
Benoit, L.B. et al. (2023) RBP Image Database: A resource for the systematic characterization of the subcellular distribution properties of human RNA binding proteins.
Nucleic Acids Res. 51 (D1): D1549-D1557. -
Bulc, M. et al. (2022) Administration of Different Doses of Acrylamide Changed the Chemical Coding of Enteric Neurons in the Jejunum in Gilts.
Int J Environ Res Public Health. 19 (21): 14514. -
Makowska, K. & Gonkowski, S. (2023) Changes Caused by Bisphenols in the Chemical Coding of Neurons of the Enteric Nervous System of Mouse Stomach.
Int J Environ Res Public Health. 20 (6) 5125. -
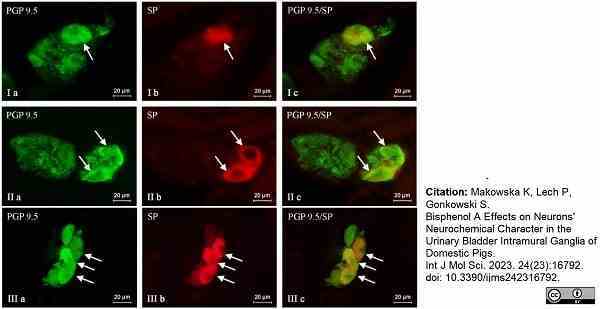
Makowska, K. et al. (2023) Bisphenol A Effects on Neurons' Neurochemical Character in the Urinary Bladder Intramural Ganglia of Domestic Pigs.
Int J Mol Sci. 24 (23): 16792. -
Ismail, H.I. & Ali, A.M. (2015) Immunohistochemical Studies on The Endocrine Cells in The Thymus of The One-Humped-Camel (Camelus dromedarius)
J Camel Pract Res. 22 (2): 251. -
Księżarczyk, M.M. & Arciszewski, M.B. (2021) Immunohistochemical study on the expressions of neuropeptides in the superficial and deep gland of the third eyelid of pigs.
Anat Histol Embryol. 50 (3): 579-87. -
Sienkiewicz, W. et al. (2022) Immunohistochemical characterization of nerve elements in porcine intrinsic laryngeal ganglia.
Pol J Vet Sci. 25 (2): 325-34. -
Rumpler, É. et al. (2021) Characterization of Kisspeptin Neurons in the Human Rostral Hypothalamus.
Neuroendocrinology. 111 (3): 249-62. -
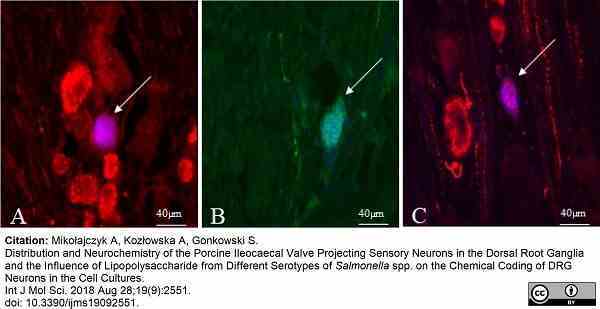
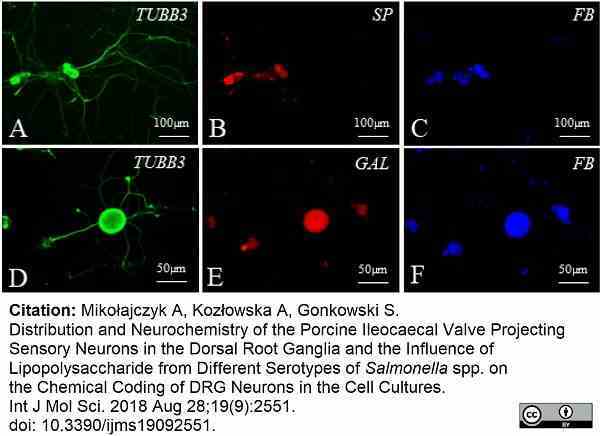
Mikołajczyk, A. et al. (2018) Distribution and Neurochemistry of the Porcine Ileocaecal Valve Projecting Sensory Neurons in the Dorsal Root Ganglia and the Influence of Lipopolysaccharide from Different Serotypes of Salmonella spp. on the Chemical Coding of DRG Neurons in the Cell Cultures.
Int J Mol Sci. 19 (9): 2551. -
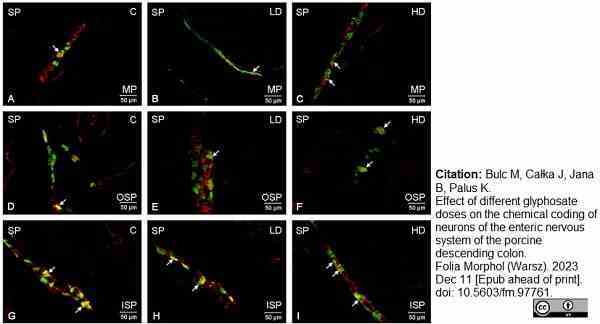
Bulc, M. et al. (2023) Effect of different glyphosate doses on the chemical coding of neurons of the enteric nervous system of the porcine descending colon.
Folia Morphol (Warsz). Dec 11 [Epub ahead of print].
- RRID
- AB_2200292
- UniProt
- P20366
- Entrez Gene
- TAC1
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
Always be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up