ErbB2 antibody | 4D5-8



Human anti ErbB2 (Trastuzumab Biosimilar)
- Product Type
- Monoclonal Antibody
- Clone
- 4D5-8
- Isotype
- IgG1
- Specificity
- ErbB2
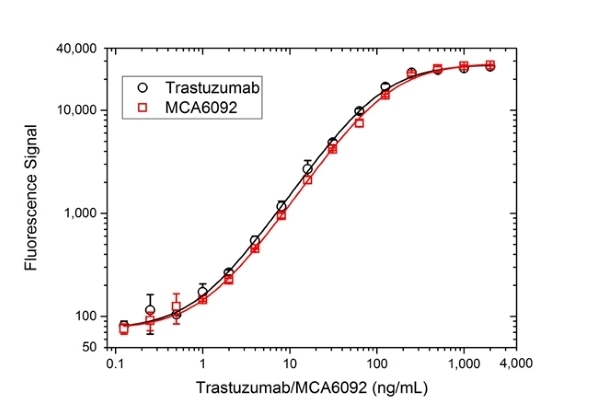
| Human Anti-ErbB2 antibody, clone 4D5-8 is a research grade biosimilar of the monoclonal antibody drug trastuzumab. This product is supplied in a research grade format, i.e. in PBS buffer with preservative, it is not manufactured or formulated in the same way as the therapeutic reference product. A preservative free version is available on request. This product is a recombinant human IgG1 kappa antibody with variable regions from the therapeutic trastuzumab antibody clone 4D5-8. Human Anti-ErbB2 antibody, clone 4D5-8 is for research use only. It is suitable as a reference standard in a pharmacokinetic (PK) bridging ELISA with anti-trastuzumab antibodies, for example HCA168 and HCA167. It can also be used as capture and detection reagent in an anti-drug antibody (ADA) bridging ELISA, with an anti-trastuzumab antibody in IgG1 format as a reference standard, for example HCA176 or HCA177. Trastuzumab, also known as Herceptin, is a drug used in the treatment of HER2 positive breast cancer and other HER2 over-expressing cancers including HER2-positive metastatic cancers of the gastrointestinal tract. Trastuzumab binds to the HER2 (or c-erbB2) proto-oncogene, an EGF receptor-like protein found on 20-30% of breast cancer cells. The binding leads to antibody mediated (complement mediated) killing of the HER2 positive cells. This product is NOT FOR THERAPEUTIC USE. View a summary of supporting anti-trastuzumab antibody products. |
- Target Species
- Human
- Product Form
- Human IgG1 (kappa) antibody - liquid
- Preparation
- Purified recombinant IgG prepared by affinity chromatography on Protein A from a mammalian cell line
- Source
- HEK
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.02% ProClin 300
- Immunogen
- A431 cells (human epidermoid carcinoma) (over)expressing EGFR
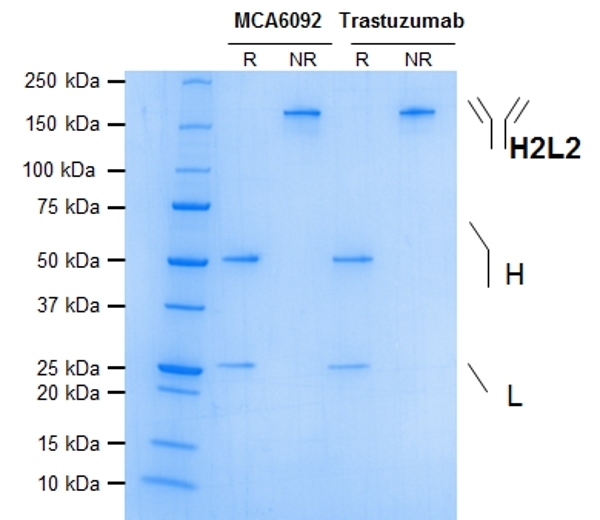
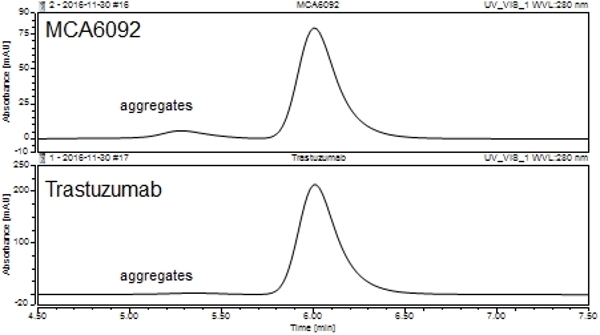
- Purity
- >98% by SDS PAGE
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
- Acknowledgements
- ProClin is a trademark of The Dow Chemical Company (“Dow”) or an affiliated company of Dow.
- Licensed Use
- For in vitro research purposes only. Research grade biosimilar. Not for use in therapeutic or diagnostic procedures for humans or animals.
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA |
Further Reading
-
Pivot, X. et al. (2015) Challenges in the implementation of trastuzumab biosimilars: an expert panel's recommendations.
Anticancer Drugs. 26 (10): 1009-16.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Human ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up