Denosumab antibody | AbD26295_hIgG1





Human anti Denosumab
- Product Type
- Monoclonal Antibody
- Clone
- AbD26295_hIgG1
- Isotype
- IgG1
- Specificity
- Denosumab
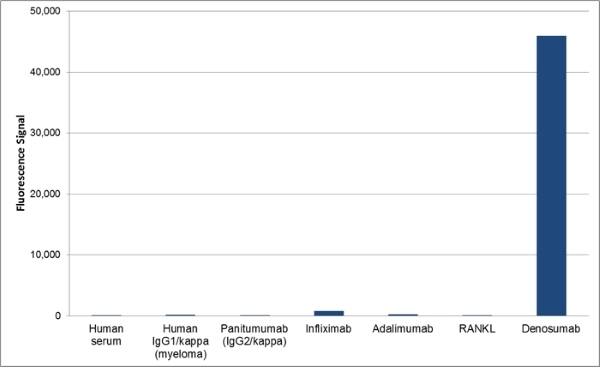
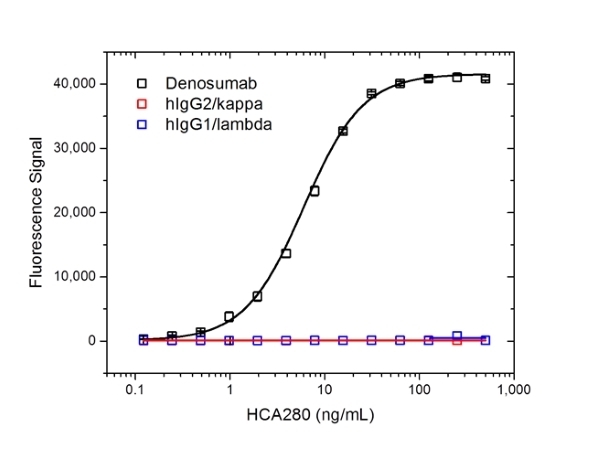
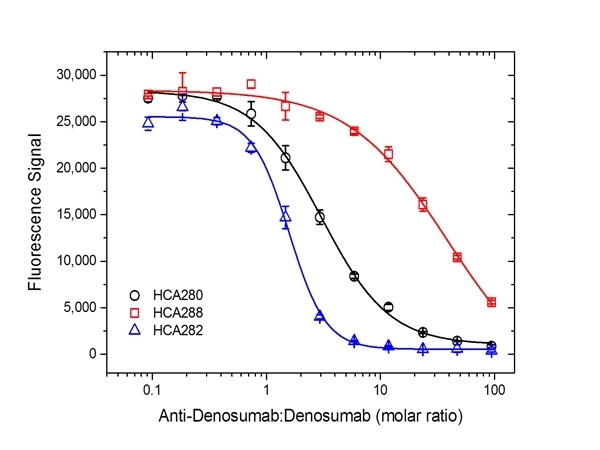
| Human Anti-Denosumab Antibody, clone AbD26295_hIgG1 is a paratope specific anti-idiotypic antibody that specifically recognizes the free human monoclonal antibody denosumab. The antibody does not recognize free RANKL (receptor activator of nuclear factor kappa-B ligand) or denosumab in complex with human RANKL and can be used to measure free denosumab and biosimilar products. Clone AbD26295_hIgG1 is a fully human recombinant monoclonal antibody with IgG1 isotype and is suitable as a reference standard in an anti-drug antibody (ADA) assay. A pair of anti denosumab antibodies can be used to develop a pharmacokinetic (PK) bridging assay to measure free drug. This antibody, in full immunoglobulin format, is recommended as the detection antibody paired with clone AbD26296 (HCA288) in monovalent Fab format as the capture antibody. Denosumab (Prolia, Xgeva) is a fully human monoclonal antibody (IgG2/kappa) for the treatment of osteoporosis, treatment-induced bone loss, bone metastases, multiple myeloma, and giant cell tumor of bone. The drug specifically binds to human RANKL a protein that acts as the primary signal to promote bone removal/resorption and prevents RANKL from activating its receptor, RANK, on the surface of osteoclasts and their precursors. View a summary of all anti-denosumab antibodies. |
|
- Product Form
- Human IgG1 antibody (kappa light chain) selected from the HuCAL phage display library and expressed in a human cell line - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A
- Source
- HKB-11
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% Sodium Azide (NaN3)
- Immunogen
- Denosumab
- Affinity
- The intrinsic affinity of the monovalent form of this antibody is KD=1.8 nM as measured by real time, label-free molecular interaction analysis on immobilized Denosumab.
- Approx. Protein Concentrations
- IgG concentration 0.5 mg/ml
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
- Acknowledgements
- This product and/or its use is covered by claims of U.S. patents, and/or pending U.S. and non-U.S. patent applications owned by or under license to Bio-Rad Laboratories, Inc. See bio-rad.com/en-us/trademarks for details.
Prolia and Xgeva are trademarks of Amgen Inc. - Licensed Use
- For in vitro research purposes and for commercial applications for the provision of in vitro testing services to support preclinical and clinical drug development. Any re-sale in any form or any other commercial application needs a written agreement with Bio-Rad.
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA |
- Technical Advice
- Recommended protocols and further information about HuCAL recombinant antibody technology can be found in the HuCAL Antibodies Technical Manual.
- ELISA
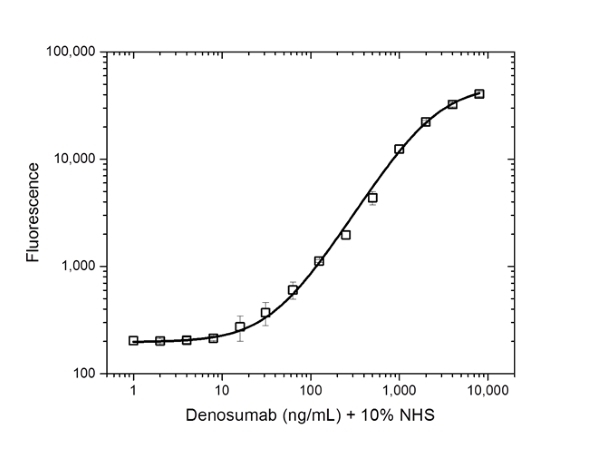
- This product may be used in direct or indirect ELISA and in an ELISA bridging assay as a detection antibody together, when conjugated to HRP, with HCA288 as the capture reagent. It is also suitable as a fully human control or calibrator antibody in an ADA bridging ELISA.
Protocol: PK bridging ELISA to measure free drug.
Protocol: ADA bridging ELISA.
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Hispec Assay Diluent | BUF049A | E IY | 50 ml | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Hispec Assay Diluent | ||||||
| Human anti Denosumab:HRP | HCA280P | E | 0.1 mg | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Human anti Denosumab:HRP | ||||||
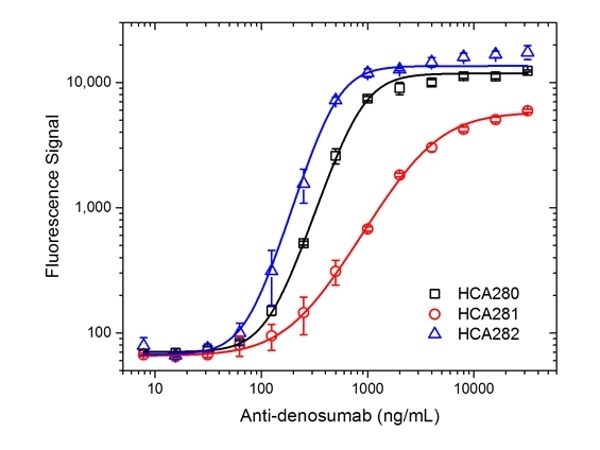
| Human anti Denosumab | HCA281 | E | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Human anti Denosumab | ||||||
| Human anti Denosumab | HCA282 | E | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Human anti Denosumab | ||||||
| Human anti Denosumab | HCA288 | E | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Human anti Denosumab | ||||||
| LYNX Rapid HRP Antibody Conjugation Kit | LNK001P | CJ | 1 Conjugation For 400µg Antibody | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | LYNX Rapid HRP Antibody Conjugation Kit | ||||||
- Synonyms
- Prolia
- Xgeva
HCA280
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
Always be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up