Western Blot Protocol: PrecisionAb

- On This Page
- Overview
- Materials
- Method
- Additional resources
s
s
s
Cancer Pathway Posters
Compiling key proteins involved in key signaling pathways: cGAS-STING, EGF R, mTOR, NF-kB, and PI3/AKT pathways.
s

Overview
PrecisionAb Antibodies have been extensively validated for western blotting. This protocol describes how to use PrecisionAb Antibodies to get the best western blotting results.
At the end of the protocol, there are details for more resources that will provide comprehensive procedures and guidance to produce successful western blots.
Materials
- Protein samples and reagents
- Lysates
- Reducing agents such as dithiothreitol (DTT) or ß-mercaptoethanol (BME)
- Electrophoresis gels, reagents, and equipment
- Phosphate Buffered Saline (PBS) containing 1% w/v BSA
- 4–15% Mini-PROTEAN® TGX Stain-Free Precast Gels (10 well, 50 µl)
- Any kD Mini-PROTEAN® TGX Stain-Free Precast Gels (10 well, 50 µl)
- Mini-PROTEAN Tetra Cell for Mini Precast Gels
- PowerPac Universal Power Supply
- Precision Plus Protein All Blue Standards Value Pack
- 1x Tris/glycine/SDS (TGS; running buffer)
- 2x Laemmli sample buffer
- Transfer membranes, reagents, and equipment
- Western blotting reagents and equipment
- Primary antibodies
- Secondary antibodies (see antibody datasheet)
- 10x Tris-buffered saline (TBS)
- 10% Tween 20
- 1x TBS + 1% casein (blocking buffer)
- 1x TBS + 0.1% Tween 20 (TBST)
- Imaging reagents and equipment
Method
-
Lysate preparation
-
Reconstitute 400 µg lysate in one of the following ways, depending on the reducing reagent used:
- If using DTT, add 190 μl H20, 200 μl 2x Laemmli Sample Buffer, and 10 μl 2 M DTT
- If using BME, add 180 μl H20, 200 μl 2x Laemmli Sample Buffer, and 20 μl BME
- Heat at 95°C for 5 min.
-
Gel Electrophoresis
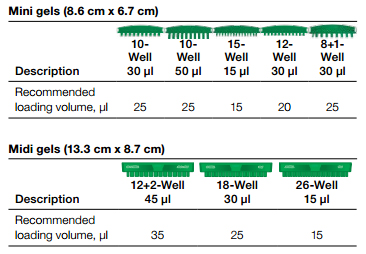
- Load the control cell lysate adjacent to your samples and the molecular weight (MW) marker (see diagram).

- The amount of control lysate loaded is dependent on the gel comb size. Please see the recommendations in the tables below.
-
Blocking
- During the validation process, we blocked for 30 min at room temperature (RT) in blocking buffer + 0.1% Tween 20. When using casein, do not block for longer than 30 min to prevent reduction in signal specificity. We recommend using casein or nonfat dried milk for blocking. If using BSA, you may notice some nonspecific bands due to its low stringency. Please see the validation protocol (bulletin 6603) for more details.
-
Incubation with Primary Antibody
-
Dilute the primary antibody 1:1,000 in 10 ml blocking buffer
- Incubate the blot in the primary antibody and blocking buffer solution at 4°C overnight with gentle agitation
- Rinse the blot with 15 ml TBST at RT for 5 min. Repeat for a total of five washes
-
Incubation with Secondary Antibody
- Rinse the blot with 15 ml TBST at RT for 5 min. Repeat for a total of five washes
- Dilute the appropriate secondary antibody in 10 ml blocking buffer according to the following table:
| Primary Antibody Host | Secondary | Bio-Rad Catalog | Recommended Dilution |
|---|---|---|---|
| Mouse | GAM-HRP | STAR207P | 1:10,000 |
| Rabbit | GAR-HRP | STAR208P | 1:10,000 |
| HuCAL | Goat anti-human IgG F(ab')2:HRP | STAR126P | 1:2,500 |
| Rat | Goat anti-rat IgG:HRP | STAR72 | 1:10,000 |
| Goat | Donkey anti-sheep/goat IgG:HRP | STAR88P | 1:10,000 |
| Sheep | Rabbit anti-sheep IgG:HRP | 5184-2504 | 1:10,000 |
-
Please refer to the antibody product page for details on the exact secondary antibody used during the validation process.
- Incubate the blot in the secondary antibody and blocking buffer solution at RT for 1 hr with gentle agitation
- Rinse the blot with 15 ml TBST at RT for 5 min. Repeat for a total of five washes. After the final wash step, keep the blot in TBST while preparing for blot detection
-
Blot Detection
-
All PrecisionAb Antibodies were validated using enhanced chemiluminescent (ECL) detection. It is important to use an ECL substrate that has good sensitivity and long signal duration, such as the Clarity Western ECL Substrate.
- Mix the Clarity Western ECL Substrate Kit components in a 1:1 ratio. Prepare 0.1 ml solution per cm2 of membrane
- Place the membrane, protein side down, in the substrate solution, and let the membrane develop for 5 min
Additional Resources
For more western blotting tips, please use the following resources

|
|
|
| Bulletin 2895 | Western blotting overview | Troubleshoot western blots |