Trichomonas Vaginalis

- On This Page
- Overview
- T. vaginalis infection
- T. vaginalis’ proteome
- Immune responses
- References
Overview
Trichomonas vaginalis is a flagellate parasitic protozoan, originally described by Alfred François Donné in 1836 as the causative agent of trichomoniasis. It is a sexually transmitted disease (STD) regarded as mild and easily curable, but its wide incidence and associated health complications, compounded by reliance on a few therapeutics beginning to encounter increasing resistance, makes it a public health problem. Additionally, the immunology of T. vaginalis infections needs further research as large knowledge gaps remain.
T. vaginalis Infection
T. vaginalis infection is the most common nonviral STD. A 2019 publication by the World Health Organization (WHO) estimated global T. vaginalis cases in 2016 at 156 million, representing almost 50% of global STDs (Rowley et al. 2019). A US study discovered infection rates of 1.8% in women and 0.5% in men aged 18–59 years (Patel et al. 2018). Carriers can be asymptomatic. Trichomoniasis is often concomitant with other STDs, mainly gonorrhea and bacterial vaginosis in women (Wølner-Hanssen et al. 1989). Additional complications, possibly due to damage to mucosal membranes, are increased acquisition of HIV and also higher HIV transmission rates (Laga et al. 1993, Sorvillo and Kerndt 1998). T. vaginalis infection is linked to adverse outcomes in pregnancy such as preterm birth, low birth weight, and premature rupture of membranes (Silver et al. 2014).
Humans are the only natural host for T. vaginalis where it infects the urogenital tract. It has an oval shape that appears pear shaped when observed in a slide mount for diagnosis. Four anterior flagella and an undulating membrane structure, that some have termed a fifth flagella, create movement. Detecting this movement is essential for direct diagnosis. This motile trophozoite form was traditionally considered to be the only form for T. vaginalis to exist in. More recent research suggests that it can also exist as a pseudocyst with internal flagella (Pereira-Neves et al. 2003, Beri et al. 2020). T. vaginalis generates ATP with hydrogenosomes, instead of mitochondria, enabling it to survive in low oxygen environments (Chose et al. 2003). Work is still ongoing to define its physiology (Lee et al. 2009).
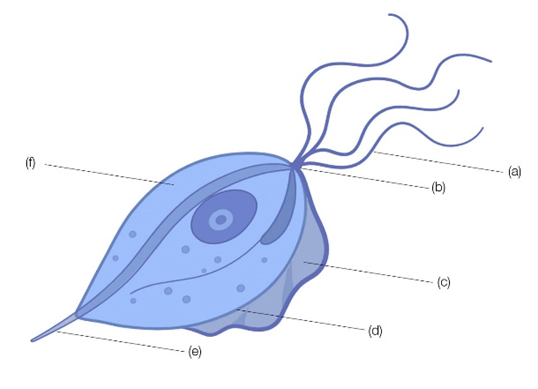
Fig. 1. Schematic drawing of Trichomonas vaginalis. (a), anterior flagellum; (b), pelto; (c), undulating membrane; (d), costa; (e), axostyle; (f), hydrogenosomes. The parasite has an average length and width of 9 to 23 and 7 µm, respectively.
T. vaginalis’ Proteome
T. vaginalis infects human epithelia by initiating adhesion, cellular damage, and immune modulation in the host tissue; it achieves that through secretion of cytolytic factors, enzymes, and interaction of parasite ligands with host cell receptors. Among them:
- T. vaginalis factor (TvF), a 250 kDa protein, induces cell rounding and clumping but not lysis (Lushbaugh et al. 1989)
- Cell-detaching factor (CDF) (200 kDa) initiates cell detachment (Garber and Lemchuk-Favel 1990)
- Cysteine proteases, located at the surface, for proteolytic breakdown of extracellular matrix, complement proteins, IgA and IgG, and induction of apoptosis in epithelial cells (Sommer et al. 2005)
- Phospholipase-A-like proteins that contribute to cytolytic activities (Lubick and Burgess 2004)
- Saposin-like proteins (SAPLIPs) that create channels in the lipid bilayer membrane. 12 SAPLIP genes (TvSaplip1 to 12) are thought to be involved (Zhai and Saier 2000)
- T. vaginalis adhesins, made up of five proteins (AP120, AP65, AP51, AP33, and AP23), that have been detected on the parasite’s surface, hence the suggestion that they are involved in adhesion (Alderete et al. 2001, Garcia and Alderete 2007). Extraordinarily, all except AP23 are also found in the hydrogenosomes and involved in metabolic functions
- Lipophosphoglycan (LPG) is one of the most abundant molecules of the T. vaginalis glycocalyx, the outer cell membrane layer of the parasite, and serves to bind galectin-1 and -3 on epithelial cells. A further mechanism for T. vaginalis to modulate immune responses (Fichorova et al. 2016)
- Bacteroides surface protein A (BspA)-like is a protein family with hundreds of members thought to be involved in cell adhesion. These have been identified through genomic analyses in T. vaginalis, but have not been characterized in detail (Noël et al. 2010, Gould et al. 2013)
- T. vaginalis macrophage migration inhibitory factor (TvMIF) binds with high affinity to the human CD74 MIF receptor, inhibits macrophage migration, and is proinflammatory. This is an immunomodulatory function, which as a side effect, has been proposed to increase the severity of prostate cancer and benign prostate hyperplasia (Twu et al. 2014)
Finally, T. vaginalis also makes use of exosomes to deliver RNA and proteins into host epithelial cells to modulate the host’s immune response. The transfer involves 4-α-glucanotransferase (Tv4AGT) which binds heparan sulfate on the epithelial cells to initiate fusion in a cholesterol and caveolin-1 dependent fashion. Endocytosis then proceeds via a clathrin-independent, lipid raft-mediated mechanism (Twu et al. 2013, Rowley et al. 2019, Rai and Johnson 2019).
Immune Responses
The mucosal immune system is the prime defense against T. vaginalis. While both adaptive and innate immune responses occur, it is the innate immune system that is more beneficial in the long term as it has been found that T. vaginalis does not produce long term immunity. During the course of infection, the trophozoites trigger an inflammatory response characterized by interleukin-8 (IL-8) and interleukin-6 (IL-6) release (Fichorova 2009, Jarrett et al. 2015). Neutrophils and macrophages also release nitric oxide (Frasson et al. 2012). While IgA is produced by B cells, it has also been shown that T. vaginalis’ proteases can cleave IgA and IgG and also have leukotoxic activity against B cells (Mercer et al. 2016). The specific immune response to T. vaginalis is further complicated by the parasite’s symbiotic interaction with Mycoplasma hominis in some cases (Rappelli et al. 2001). T. vaginalis can also be infected with one or more double-stranded RNA (dsRNA) viruses which have been provisionally assigned to the Totiviridae family of dsRNA viruses (Goodman et al. 2011).
In all, while progress has been made to elucidate the proteome of T. vaginalis, its pathogenesis and host immune responses remain poorly understood. The lack of an animal model has also hindered research and vaccine development.
Trichomonas Vaginalis Antibodies
| Description | Target | Format | Clone | Applications | Citations | Code |
|---|
References
- Alderete JF et al. (2001). Enzymes on microbial pathogens and Trichomonas vaginalis: molecular mimicry and functional diversity. Cell Microbiol. 3(6), 359-370.
- Beri D et al. (2020). Demonstration and characterization of cyst-like structures in the life cycle of Trichomonas vaginalis. Front Cell Infect Microbiol. 9, 430.
- Chose O et al. (2003). Cell death in protists without mitochondria. Ann N Y Acad Sci. 1010, 121-125.
- Fichorova RN (2009). Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J Reprod Immunol. 83(1-2), 185-189.
- Fichorova RN et al. (2016). Trichomonas vaginalis Lipophosphoglycan exploits binding to galectin-1 and -3 to modulate epithelial immunity. J Biol Chem. 291(2), 998-1013.
- Frasson AP et al. (2012). Involvement of purinergic signaling on nitric oxide production by neutrophils stimulated with Trichomonas vaginalis. Purinergic Signal. 8(1), 1-9.
- Garber G E and Lemchuk-Favel LT (1990). Association of production of cell-detaching factor with the clinical presentation of Trichomonas vaginalis. J Clin Microbiol. 28(11), 2415-2417.
- Garcia AF and Alderete J (2007). Characterization of the Trichomonas vaginalis surface-associated AP65 and binding domain interacting with trichomonads and host cells. BMC Microbiol. 7, 116.
- Goodman RP et al. (2011). Clinical isolates of Trichomonas vaginalis concurrently infected by strains of up to four Trichomonasvirus species (Family Totiviridae). J Virol. 85(9), 4258-4270.
- Gould SB et al. (2013). Deep sequencing of Trichomonas vaginalis during the early infection of vaginal epithelial cells and amoeboid transition. Int J Parasitol. 43(9), 707-719.
- Jarrett OD et al. (2015). T. vaginalis infection is associated with increased IL-8 and TNFr1 levels but with the absence of CD38 and HLADR activation in the cervix of ESN. PloS one. 10(6), e0130146.
- Laga M et al. (1993). Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 7(1), 95-102.
- Lee KE et al. (2009). Three-dimensional structure of the cytoskeleton in Trichomonas vaginalis revealed new features. J Electron Microsc. 58(5), 305-313.
- Lubick KJ and Burgess DE (2004). Purification and analysis of a phospholipase A2-like lytic factor of Trichomonas vaginalis. Infection Immun. 72(3), 1284-1290.
- Lushbaugh WB et al. (1989). Characterization of a secreted cytoactive factor from Trichomonas vaginalis. Am J Trop Med Hyg. 41(1), 18–28.
- Mercer F et al. (2016). Leukocyte lysis and cytokine induction by the human sexually transmitted parasite Trichomonas vaginalis. PLoS Negl Trop Dis. 10(8), e0004913.
- Noël CJ et al. (2010). Trichomonas vaginalis vast BspA-like gene family: evidence for functional diversity from structural organisation and transcriptomics. BMC genomics. 11, 99.
- Patel EU et al. (2018). Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin. Infect. Dis. 67(2), 211-217.
- Pereira-Neves A et al. (2003). Pseudocysts in Trichomonads – new insights. Protist. 154, 313-329.
- Rai AK and Johnson PJ (2019). Trichomonas vaginalis extracellular vesicles are internalized by host cells using proteoglycans and caveolin-dependent endocytosis. Proc Natl Acad Sci U S A. 116(43), 21354-21360.
- Rappelli P et al. (2001). Mycoplasma hominis and Trichomonas vaginalis symbiosis: multiplicity of infection and transmissibility of M. hominis to human cells. Arch Microbiol. 175(1), 70-74.
- Rowley J et al. (2019). Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bulletin of the World Health Organization. 97(8), 548-562P.
- Silver BJ et al. (2014). Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sex Transm Dis. 41(6), 369-376.
- Sommer U et al. (2005). Identification of Trichomonas vaginalis cysteine proteases that induce apoptosis in human vaginal epithelial cells. J Biol Chem. 280(25), 23853-23860.
- Sorvillo F and Kerndt P (1998). Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet. 351(9097), 213-214.
- Twu O et al. (2013) Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host∶ parasite interactions. PLOS Pathog. 9(7), e1003482.
- Twu O et al. (2014). Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proc Natl Acad Sci U S A. 111(22), 8179-8184.
- Wølner-Hanssen P et al. (1989). Clinical manifestations of vaginal trichomoniasis. JAMA. 261(4), 571-576.
- Zhai Y and Saier MH Jr (2000). The amoebapore superfamily. Biochim Biophys Acta. 1469(2), 87-99.





