s
Step by step protocol for mini spin columns
Resin plug loading
1. Load the pre-packed resin Mini plug containing immobilized IMAC resin into the Proteus spin column barrel using the Mini insertion tool as shown on page 14.
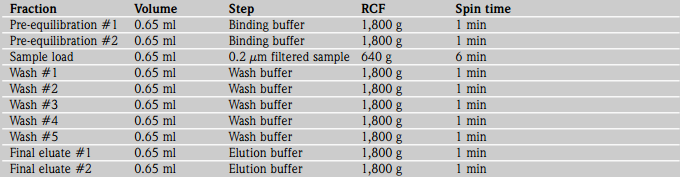
Pre-equilibration (Total spin times = 2 mins)
2. Equilibrate the IMAC spin column with 0.65 ml binding buffer pH 7.4 (10 mM imidazole) by centrifuging the spin column at 1,800 g (4,400 rpm in a Heraeus Biofuge Pico or 5,000 rpm in a Sanyo MSE Micro Centaur) for 1 min. Repeat this pre-equilibration step with 0.65 ml binding buffer, pH 7.4 at 1,800 g for 1 min.
Clarification of sample
3. Filter 1 ml sample through a 0.2 µm pore size syringe filter to remove any cellular debris, precipitating protein complexes just prior to sample loading.
Sample loading (Total spin time = 6 mins)
4. Pipette up to 0.65 ml filtered cleared lysate into the spin column. Centrifuge the spin column at 640 g (2,600 rpm in a Heraeus Biofuge Pico or 3,000 rpm in a Sanyo MSE Micro Centaur) for 6 mins. It may be necessary to increase the spin time or spin speed if any sample remains above the plug.
N.B. In some circumstances, you may wish to re-apply the sample wash back through the spin column before the wash step in order to increase the residence time between the target protein and the resin plug for efficient binding.
Washing (Total spin time = 5 mins)
5. Wash the spin columns up to five times with 0.65 ml wash buffer, pH 7.4 (30 mM imidazole) to remove non-tagged proteins with no affinity for the immobilized metal ion by centrifuging the spin columns for 1 min at 1,800 g (4,400 rpm in a Heraeus Biofuge Pico or 5,000 rpm in a Sanyo MSE Micro Centaur). The washes should be collected for analysis. As imidazole absorbs UV radiation at 280 nm, we recommend that the wash buffer is used as the reference solution for auto-zeroing the UV-Vis spectrophotometer if imidazole is used to elute the target protein from the spin columns.
Elution (Total spin times = 2 mins)
6. Elute the bound His-tagged protein with 0.65 ml elution buffer pH 7.4 (300 mM imidazole) directly into a fresh centrifuge tube by centrifuging the spin columns for 1 min at 1,800 g. The eluate should be collected for further analysis. Repeat the above elution procedure faithfully to ensure complete recovery of all recombinant proteins.
N.B. Check the protein content of each eluted fraction before pooling them. Otherwise, you risk diluting a concentrated, purified sample.
Desalting and concentrating
7. Imidazole and any residual metal ions should be removed by diafiltration using ultrafiltration concentrators or rapid dialysis against an appropriate buffer for your downstream application. Otherwise, the imidazole may strip the metal ion from a metalloprotein of interest or the target protein may irreversibly precipitate out of solution when stored at –20 °C or –80 °C.

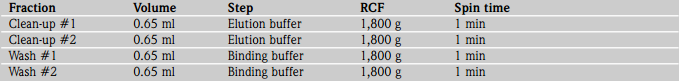
Regeneration of the IMAC mini plug
8. Wash the Mini plugs twice with 0.65 ml elution buffer by centrifuging the spin columns at 1,800 g for 1 min. Then wash the plugs twice with 0.65 ml binding buffer by centrifuging the spin columns at 1,800 g for 1 min. Proceed to the pre-equilibration step of another bindwash-elute cycle if the plugs are to be re-used immediately. After regeneration, plugs can also be stored, without their end caps, in 0.1 % sodium azide (made up in distilled water) at 2-8 °C until further use.
* If 1 spin column is to be used, ensure that the spin column is counterbalanced in the microfuge with a microcentrifuge tube filled with the correct level of water
Easy to read mini purification protocol:
Easy to read mini regeneration protocol: