Western Blot: Antibody Considerations
Overview
One of the critical features of any successful Western blot is the highly specific interaction between an antibody and an antigen. The antigen, usually a protein or peptide, is the target of the antibody. The precise point of interaction is between a small region of the antigen, an epitope, and the recognition sites found on the arms of the antibody molecule.
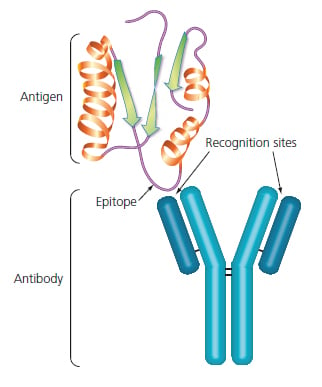
Figure 3: Antibody-Antigen interaction
Monoclonals vs. Polyclonals
The antibodies used to detect the target protein in a Western blot will be either monoclonal or polyclonal. Both types of antibody are typically created when an antigen, usually a protein or peptide, is injected into an animal and its immune system responds by producing antibodies specifically targeted against that antigen (or more precisely to various epitopes found on that antigen).
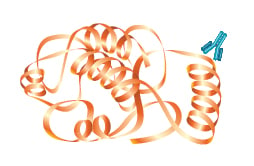
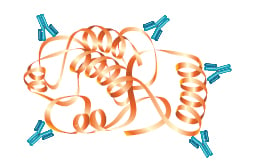
Polyclonal antibodies consist of a mixed pool of immunoglobulin molecules that bind to several different epitopes found on a single antigen. Polyclonals are usually produced in rabbits, donkeys, sheep, and goats, and are purified from serum. In contrast, monoclonal antibodies bind to a single epitope within a target antigen.
They are composed of homogeneous cloned immunoglobulin molecules, rather than the heterogeneous antibody mixture typical of polyclonals. Monoclonals are made by fusing antibody producing cells from the spleen of the immunized animal (usually a rat or mouse) with an immortalized cell line to produce single specificity antibodies that can be purified from tissue culture supernatant. Both monoclonals and polyclonals are used in Western blotting, and offer various advantages and disadvantages that are summarized in the accompanying table.
Comparison of Monoclonal and Polyclonal Antibodies
| Monoclonal | Polyclonal |
|---|---|
| Specificity for a single epitope. | Varying specificities to multiple epitopes. |
|
|
| Identifies whether a particular region of a protein is present | Identifies the entire target protein via binding at multiple sites. Since multiple epitopes are targeted, there is a higher likelihood of detection of the target |
| May cross-react with other proteins that share this epitope, such as isomers or common motifs | Higher background and cross-reactivity possible due to detection of multiple epitopes, any of which may be shared by related proteins |
| Usually less sensitive since only a single antibody molecule binds to each target | More sensitive because signal is amplified through the binding of several antibodies per target |
| More expensive to produce initially, but available in an unlimited supply | Less expensive to produce initially, but supply is limited to immunized animal(s). There will be greater variability between preparations |
Genetically Engineered Antibodies
In addition to traditional monoclonal and polyclonal antibodies targeted against specific proteins, there are other means of antibody generation and protein detection available as the result of numerous advances in genetic engineering technology. It is now possible to create and produce antibodies using fully in vitro techniques such as phage display in conjunction with highly complex libraries which represent the vast array of potential antibody binding regions. At Bio-Rad, we routinely produce human recombinant monoclonal antibodies for research, clinical, and diagnostic applications with our HuCAL® Technology
Epitope Tags
If there are no antibodies available to the protein of interest, it is still possible to carry out a range of immunodetection techniques, including Western blotting, by using epitope tags and matched epitope tag antibodies.
This elegant strategy works by adding a small sequence of DNA that codes for a known antigenic epitope during cloning of the protein of interest. Since matched antibodies already exist that will specifically bind to this epitope, the target protein can be detected because it also expresses the appropriate epitope. Therefore, immunodetection can be carried out quickly and without the need to wait for the generation of unique antibodies to a newly identified target protein. This technology is also of significant benefit when working in organisms where few specific antibodies are readily available.
One downside to this technology is that the target protein is altered by the addition of tag, and thus it is not identical to native forms of the protein. There are a wide variety of epitope tag antibodies available, including: His-6 antibody (MCA1396), V5-Tag antibody (MCA1360), c-myc antibody (MCA2200), and others which are supplied by Bio-Rad.
Epitope tag antibodies are available with a range of common antibody labels allowing one to switch experimental techniques or detection systems without having to modify the target protein. In the Western blots shown below, myc-tagged KSr (Kinase Suppressor of ras) is detected through the use of either an anti-myc antibody or an anti-KSr antibody.
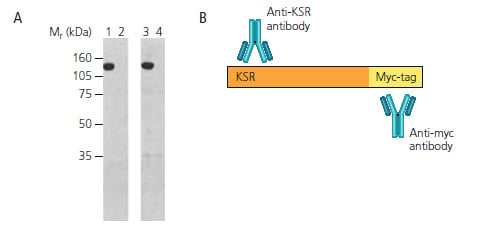
Figure 4: Western Blot and Model of Myc-tagged KSR. PANEL A: Myc-tagged murine KSr expressed in COS cells is detected using either anti-KSr antibody (MCA2106) in lanes 1&2, or anti-myc epitope antibody (MCA2200) in lanes 3 & 4. Lanes 1 & 3: myc-KSr vector. Lanes 2 & 4: vector alone control . Note that an identical band is produced with both antibodies. PANEL B: KSr target protein with c-myc epitope tag. KSr-myc fusion is detectible by both anti-KSr antibodies and anti-myc antibodies.
Bio-Rad’s PrecisionAb Antibody Range - Validation in Western Blotting
We are committed to providing our customers with reliable research tools, and are constantly raising our standards for antibody quality. The PrecisionAb Antibody range, rigorously tested in western blotting, for specificity and reproducibility, is designed to help researchers produce the highest-quality western blotting data.
Western Blotting Validated AntibodiesLearn More about PrecisionAb for Western Blotting
| Why Use a Western Blot? | Chapter 2: Samples, Gels, and Blotting |





