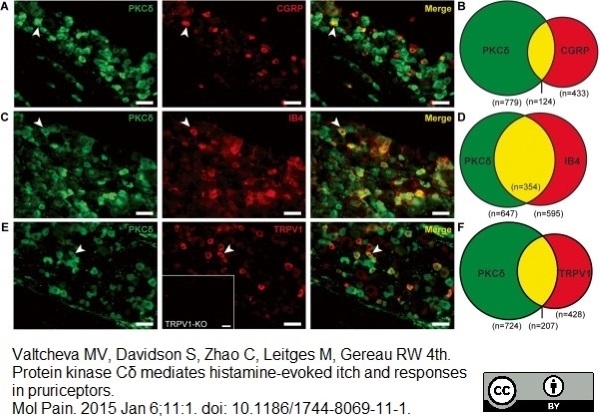
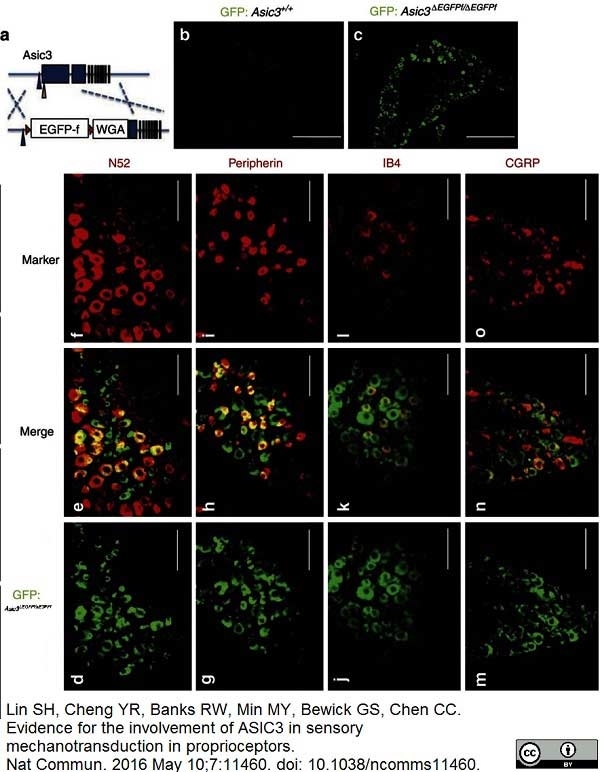
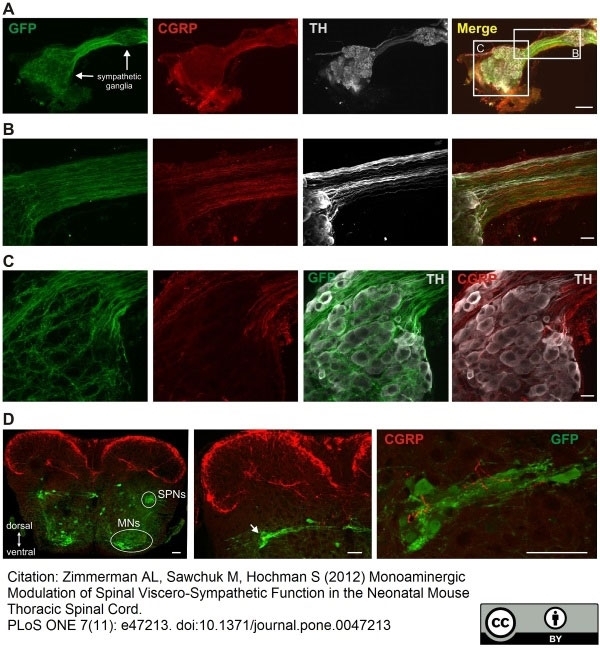
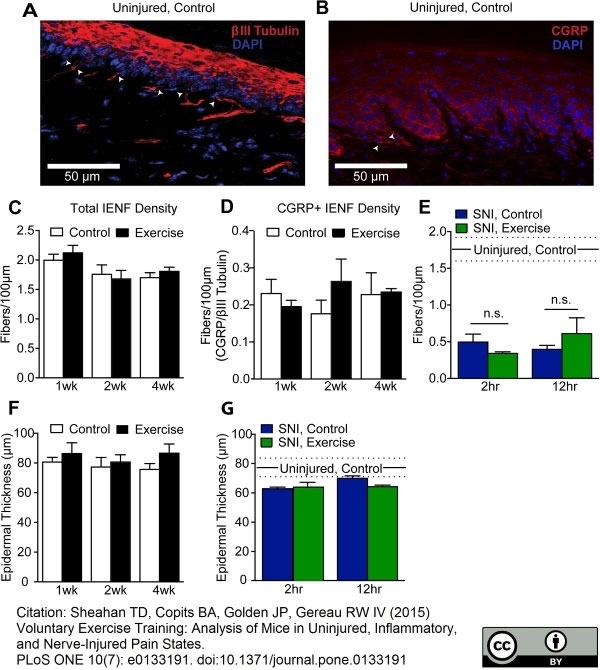
CGRP antibody
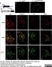





Goat anti Rat Calcitonin Gene-Related Peptide
- Product Type
- Polyclonal Antibody
- Isotype
- Polyclonal IgG
- Specificity
- CGRP
| Goat anti Rat Calcitonin Gene-Related Peptide antibody recognizes Calcitonin gene-related peptide, also known as CGRP, a neuropeptide that acts as a vasodilator and plays a role in in the pathophysiology of migraine (Recober & Russo 2009). Goat anti Rat Calcitonin Gene-Related Peptide antibody reacts with both the whole molecule (amino acids 1-37) and the C-terminal fragment (23-37). |
- Target Species
- Rat
- Species Cross-Reactivity
-
Target Species Cross Reactivity Mouse Guinea Pig Emu - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified Ig - liquid
- Preparation
- Purified Ig prepared by affinity chromatography on Protein G
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
0.09% Sodium Azide (NaN3) - Immunogen
- Synthetic rat Tyr-CGRP (23-37) conjugated to gamma globulin.
- Approx. Protein Concentrations
- IgG concentration 5.0 mg/ml
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
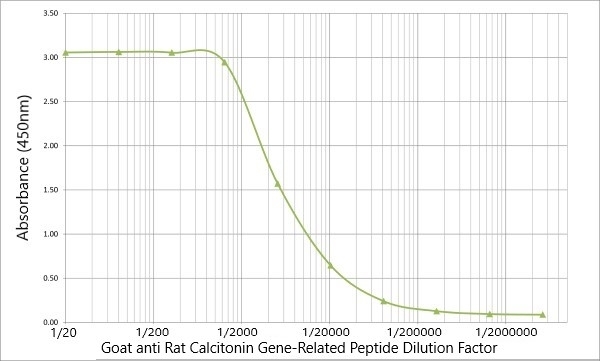
| ELISA | 1/500 | 1/2500 | |
| Immunofluorescence | |||
| Immunohistology - Frozen | |||
| Immunohistology - Paraffin | |||
| Immunohistology - Resin |
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Rabbit anti Goat IgG (Fc):FITC | STAR122F | F | 1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rabbit anti Goat IgG (Fc):FITC | ||||||
| Rabbit anti Goat IgG (Fc):HRP | STAR122P | C E WB | 1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rabbit anti Goat IgG (Fc):HRP | ||||||
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Antigen Retrieval Buffer, pH8.0 | BUF025A | P | 500 ml | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Antigen Retrieval Buffer, pH8.0 | ||||||
References for CGRP antibody
-
Collins, J.J. et al. (2000) Distribution and origin of secretoneurin-immunoreactive nerves in the female rat uterus.
Neuroscience. 95 (1): 255-64. -
Chetty, R. et al. (2006) Pancreatic endocrine tumour with ductules: further observations of an unusual histological subtype.
Pathology. 38 (1): 5-9. -
Weir, K.A. and Lunam, C.A. (2006) Immunohistochemical study of cutaneous nerves in the emu.
Cell Tissue Res. 326: 697-705. -
Brock, J.A. et al. (2007) Postnatal androgen deprivation dissociates the development of smooth muscle innervation from functional neurotransmission in mouse vas deferens.
J Physiol. 581: 665-78. -
Marchant, N.J. et al. (2007) Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division.
J Comp Neurol. 504: 702-15. -
Pitzer, C. et al. (2008) Granulocyte-colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis.
Brain. 131: 3335-47. -
Ikeda, E. et al. (2009) Fully functional bioengineered tooth replacement as an organ replacement therapy.
Proc Natl Acad Sci U S A. 106: 13475-80. -
Iliff, J.J. et al. (2009) Epoxyeicosanoids as mediators of neurogenic vasodilation in cerebral vessels.
Am J Physiol Heart Circ Physiol. 296: H1352-63.
View The Latest Product References
-
Yu, W.M. et al. (2009) Disruption of laminin in the peripheral nervous system impedes nonmyelinating Schwann cell development and impairs nociceptive sensory function.
Glia. 57: 850-9. -
Golden, J.P. et al. (2010) RET signaling is required for survival and normal function of nonpeptidergic nociceptors.
J Neurosci. 30: 3983-94. -
Iliff, J.J. et al. (2010) Epoxyeicosatrienoic acids are endogenous regulators of vasoactive neuropeptide release from trigeminal ganglion neurons.
J Neurochem. 115: 1530-42. -
Fan, W. et al. (2010) Structural and cellular features in metaphyseal and diaphyseal periosteum of osteoporotic rats.
J Mol Histol. 41: 51-60. -
Gnanamanickam, G.J. and Llewellyn-Smith, I.J. (2011) Innervation of the rat uterus at estrus: a study in full-thickness, immunoperoxidase-stained whole-mount preparations.
J Comp Neurol. 519: 621-43. -
Tague, S.E. and Smith, P.G. (2011) Vitamin D receptor and enzyme expression in dorsal root ganglia of adult female rats: modulation by ovarian hormones.
J Chem Neuroanat. 41: 1-12. -
Hamed, K. et al. (2011) Changes in cutaneous innervation in patients with chronic pain after burns.
Burns. 37: 631-7 -
Zou, M. et al. (2012) Brn3a/Pou4f1 regulates dorsal root ganglion sensory neuron specification and axonal projection into the spinal cord.
Dev Biol. 364: 114-27. -
Zimmerman, A.L. et al. (2012) Monoaminergic Modulation of Spinal Viscero-Sympathetic Function in the Neonatal Mouse Thoracic Spinal Cord
PLoS One. 7: e47213. -
Drummond, E.S. et al. (2014) Increased expression of cutaneous α1-adrenoceptors after chronic constriction injury in rats.
J Pain. 15 (2): 188-96. -
Drummond, P.D. et al. (2014) Upregulation of α1-adrenoceptors on cutaneous nerve fibres after partial sciatic nerve ligation and in complex regional pain syndrome type II.
Pain. 155: 606-16. -
Li, Z. et al. (2014) Activation of MrgC receptor inhibits N-type calcium channels in small-diameter primary sensory neurons in mice.
Pain. 155 (8): 1613-21. -
Park, S.I. et al. (2015) Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics.
Nat Biotechnol. 33 (12): 1280-1286. -
O'Brien, D.E. et al. (2015) ERK2 Alone Drives Inflammatory Pain But Cooperates with ERK1 in Sensory Neuron Survival.
J Neurosci. 35 (25): 9491-507. -
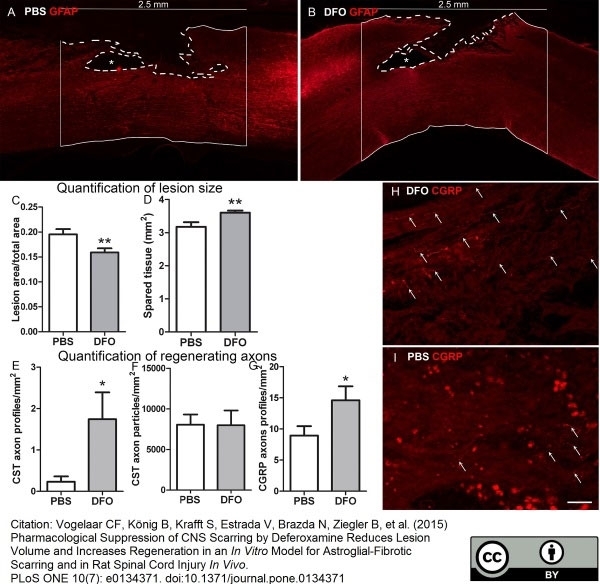
Vogelaar CF et al. (2015) Pharmacological Suppression of CNS Scarring by Deferoxamine Reduces Lesion Volume and Increases Regeneration in an In Vitro Model for Astroglial-Fibrotic Scarring and in Rat Spinal Cord Injury In Vivo.
PLoS One. 10 (7): e0134371. -
Sheahan, T.D. et al. (2015) Voluntary Exercise Training: Analysis of Mice in Uninjured, Inflammatory, and Nerve-Injured Pain States.
PLoS One. 10 (7): e0133191. -
Huang, A.Y. & Wu, S.Y. (2015) Calcitonin Gene-Related Peptide Reduces Taste-Evoked ATP Secretion from Mouse Taste Buds.
J Neurosci. 35 (37): 12714-24. -
Watanabe, M. et al. (2015) Expression and Regulation of Cav3.2 T-Type Calcium Channels during Inflammatory Hyperalgesia in Mouse Dorsal Root Ganglion Neurons.
PLoS One. 10 (5): e0127572. -
Chucair-Elliott, A.J. et al. (2015) Degeneration and regeneration of corneal nerves in response to HSV-1 infection.
Invest Ophthalmol Vis Sci. 56 (2): 1097-107. -
Van Steenwinckel, J. et al. (2015) Stromal cell-derived CCL2 drives neuropathic pain states through myeloid cell infiltration in injured nerve.
Brain Behav Immun. 45: 198-210. -
Payne, S.C. et al. (2015) Regeneration of sensory but not motor axons following visceral nerve injury.
Exp Neurol. 266: 127-42. -
Valtcheva, M.V. et al. (2015) Enhanced nonpeptidergic intraepidermal fiber density and an expanded subset of chloroquine-responsive trigeminal neurons in a mouse model of dry skin itch.
J Pain. 16 (4): 346-56. -
Chucair-Elliott, A.J. et al. (2015) Degeneration and regeneration of corneal nerves in response to HSV-1 infection.
Invest Ophthalmol Vis Sci. 56 (2): 1097-107. -
Marvaldi L et al. (2015) Enhanced axon outgrowth and improved long-distance axon regeneration in sprouty2 deficient mice.
Dev Neurobiol. 75 (3): 217-31. -
Wong, A.W. et al. (2015) Neurite outgrowth in normal and injured primary sensory neurons reveals different regulation by nerve growth factor (NGF) and artemin.
Mol Cell Neurosci. 65: 125-34. -
Lin, S.H. et al. (2016) Evidence for the involvement of ASIC3 in sensory mechanotransduction in proprioceptors.
Nat Commun. 7: 11460. -
Kim, Y.S. et al. (2016) Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain.
Neuron. 91 (5): 1085-96. -
Takahashi, N. et al. (2016) Morphology of P2X3-immunoreactive nerve endings in the rat laryngeal mucosa.
Histochem Cell Biol. 145 (2): 131-46. -
Takiguchi, M. et al. (2018) Neonatal spinal injury induces de novo projections of primary afferents to the lumbosacral intermediolateral nucleus in rats.
IBRO Rep. 4: 1-6. -
Sun, Y. et al. (2020) Somatostatin neurons in the central amygdala mediate anxiety by disinhibition of the central sublenticular extended amygdala.
Mol Psychiatry. Oct 01 [Epub ahead of print]. -
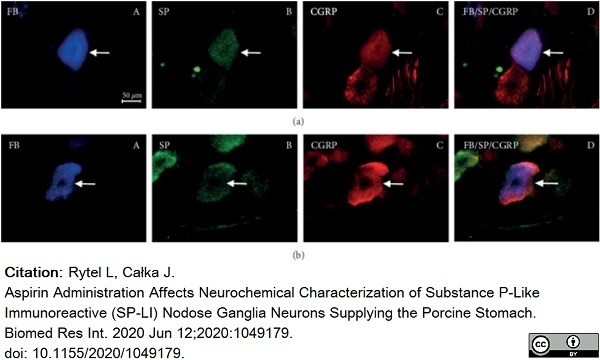
Rytel, L. & Całka, J. (2020) Aspirin Administration Affects Neurochemical Characterization of Substance P-Like Immunoreactive (SP-LI) Nodose Ganglia Neurons Supplying the Porcine Stomach.
Biomed Res Int. 2020: 1049179. -
Morellini, N. et al. (2020) Decreased neural expression of the noradrenaline transporter in the papillary dermis after partial sciatic nerve lesion.
J Chem Neuroanat. 107: 101806. -
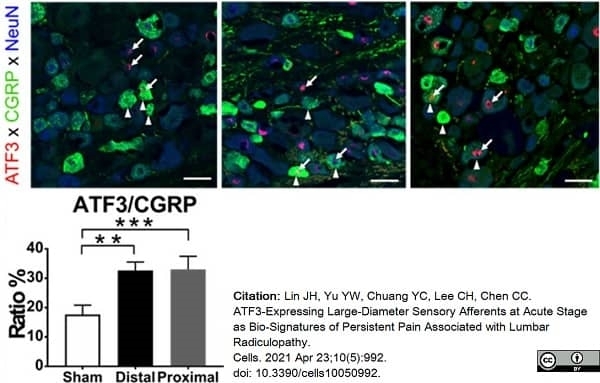
Lin, J.H. et al. (2021) ATF3-Expressing Large-Diameter Sensory Afferents at Acute Stage as Bio-Signatures of Persistent Pain Associated with Lumbar Radiculopathy.
Cells. 10 (5): 992 -
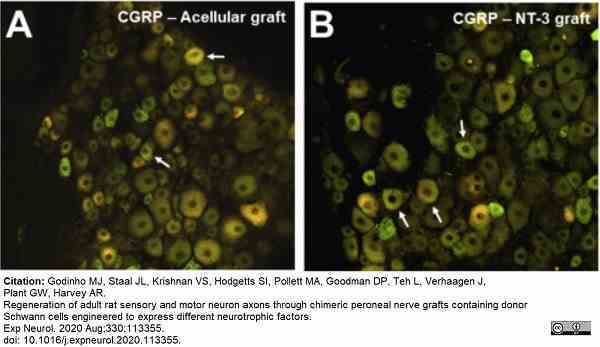
Godinho, M.J. et al. (2020) Regeneration of adult rat sensory and motor neuron axons through chimeric peroneal nerve grafts containing donor Schwann cells engineered to express different neurotrophic factors.
Exp Neurol. 330: 113355. -
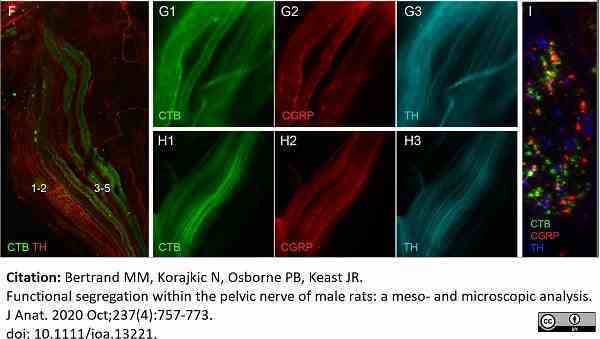
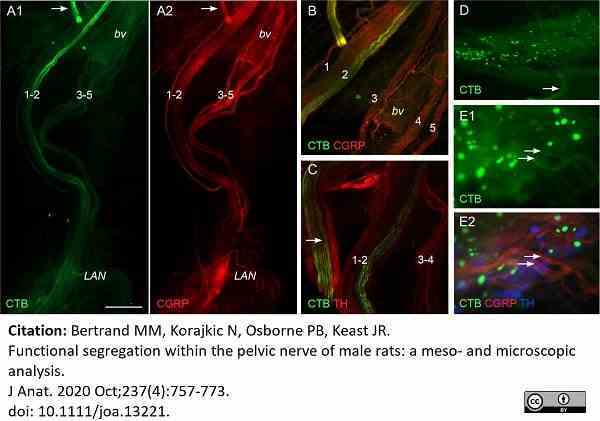
Bertrand, M.M. et al. (2020) Functional segregation within the pelvic nerve of male rats: a meso- and microscopic analysis.
J Anat. 237 (4): 757-773.
- RRID
- AB_2290729
- UniProt
- P01256
- Entrez Gene
- Calca
- GO Terms
- GO:0005576 extracellular region
- GO:0005179 hormone activity
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Rat ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up