CD31 antibody | LCI-4



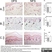




Mouse anti Pig CD31:RPE
- Product Type
- Monoclonal Antibody
- Clone
- LCI-4
- Isotype
- IgG1
- Specificity
- CD31
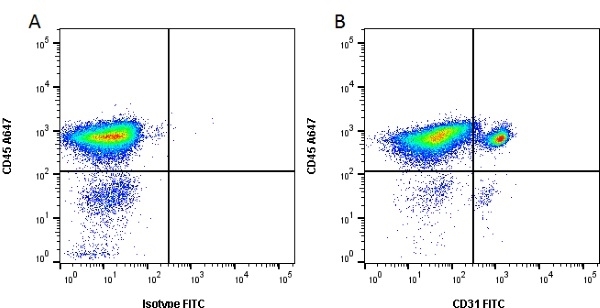
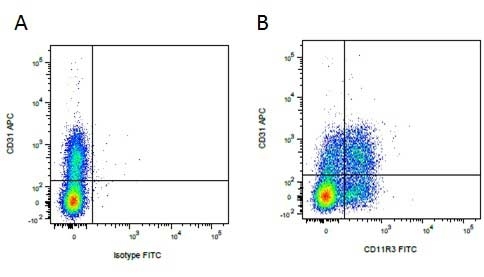
| Mouse anti Pig CD31, clone LCI-4 recognizes porcine CD31, also known as Platelet endothelial cell adhesion molecule (PECAM-1). CD31 is constitutively expressed by platelets, monocytes and some lymphocytes, it is expressed by endothelial cells at a level, an order of magnitude greater that of other cell types (Fawcwett et al.1995). The extracellular region contains six Ig-like domains. Mouse anti Pig CD31, clone LCI-4 is cross reactive with human CD31 and binds to the 5th extracellular Ig domain, proximal to the transmembrane region as demonstrated by human CD31 domain deletion mutants (Nasu et al.1999). Mouse anti Pig CD31, clone LCI-4 immunoprecipitates a protein of ~130 kDa from lysates of porcine aortic endothelial cells and is strongly expressed at cell junctions (Nasu et al. 1999). |
- Target Species
- Pig
- Species Cross-Reactivity
-
Target Species Cross Reactivity Human Mouse - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG conjugated to R. Phycoerythrin (RPE) - lyophilized
- Reconstitution
-
MCA1746PET: Reconstitute with 0.25ml distilled water
Care should be taken during reconstitution as the protein may appear as a film at the bottom of the vial. Bio-Rad recommend that the vial is gently mixed after reconstitution. - MCA1746PE: Reconstitute with 1 ml distilled water
- Preparation
- Purified IgG prepared by affinity chromatography from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
1% bovine serum albumin
5% sucrose - Immunogen
- Porcine CD31/human IgGFc fusion protein.
- Max Ex/Em
-
Fluorophore Excitation Max (nm) Emission Max (nm) RPE 488nm laser 496 578 - Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
DO NOT FREEZE
This product should be stored undiluted. This product is photosensitive and should be protected from light. Should this product contain a precipitate we recommend microcentrifugation before use.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | Neat |
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells or 100μl whole blood
How to Use the Spectraviewer
Watch the Tool Tutorial Video ▸- Start by selecting the application you are interested in, with the option to select an instrument from the drop down menu or create a customized instrument
- Select the fluorophores or fluorescent proteins you want to include in your panel to check compatibility
- Select the lasers and filters you wish to include
- Select combined or multi-laser view to visualize the spectra
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG1 Negative Control:RPE | MCA928PE | F | 100 Tests | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Mouse IgG1 Negative Control:RPE | ||||||
References for CD31 antibody
-
Nasu, K. et al. (1999) Alpha-galactosyl-mediated activation of porcine endothelial cells: studies on CD31 and VE-cadherin in adhesion and signaling.
Transplantation. 68: 861-7. -
Evans, P.C. et al. (2001) Signaling through CD31 protects endothelial cells from apoptosis.
Transplantation. 71 (3): 343-4. -
Campos, E. et al. (2004) In vitro effect of classical swine fever virus on a porcine aortic endothelial cell line
Vet Res. 35: 625-33. -
Waksman, R. et al. (2006) Intracoronary photodynamic therapy reduces neointimal growth without suppressing re-endothelialisation in a porcine model.
Heart. 92: 1138-44. -
Iohara, K. et al. (2008) A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp.
Stem Cells. 26 (9): 2408-18. -
Katchman, H. et al. (2008) Embryonic porcine liver as a source for transplantation: advantage of intact liver implants over isolated hepatoblasts in overcoming homeostatic inhibition by the quiescent host liver.
Stem Cells. 26: 1347-55. -
Tchorsh-Yutsis, D. et al. (2009) Pig embryonic pancreatic tissue as a source for transplantation in diabetes: transient treatment with anti-LFA1, anti-CD48, and FTY720 enables long-term graft maintenance in mice with only mild ongoing immunosuppression.
Diabetes. 58: 1585-94. -
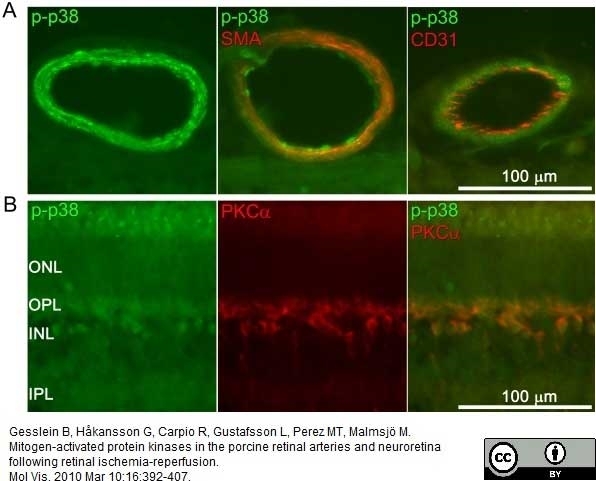
Gesslein, B. et al. (2010) Mitogen-activated protein kinases in the porcine retinal arteries and neuroretina following retinal ischemia-reperfusion.
Mol Vis. 16: 392-407.
View The Latest Product References
-
Gyöngyösi, M. et al. (2010) Differential effect of ischaemic preconditioning on mobilisation and recruitment of haematopoietic and mesenchymal stem cells in porcine myocardial ischaemia-reperfusion.
Thromb Haemost. 104 (2): 376-84. -
Poirier, N. et al. (2010) Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation.
Sci Transl Med. 2 (17): 17ra10. -
Graham, J.J. et al. (2010) Long-term tracking of bone marrow progenitor cells following intracoronary injection post-myocardial infarction in swine using MRI.
Am J Physiol Heart Circ Physiol. 299: H125-33. -
Chitalia, V.C. et al. (2011) Matrix-embedded endothelial cells are protected from the uremic milieu.
Nephrol Dial Transplant. 26: 3858-65. -
Sokoli, .A. et al. (2013) Mycoplasma suis infection results endothelial cell damage and activation: new insight into the cell tropism and pathogenicity of hemotrophic mycoplasma.
Vet Res.44: 6. -
Kang, S.D. et al. (2013) Isolation of functional human endothelial cells from small volumes of umbilical cord blood.
Ann Biomed Eng. 41 (10): 2181-92. -
Azimzadeh, A.M. et al. (2014) Development of a consensus protocol to quantify primate anti-non-Gal xenoreactive antibodies using pig aortic endothelial cells.
Xenotransplantation. 21 (6): 555-66. -
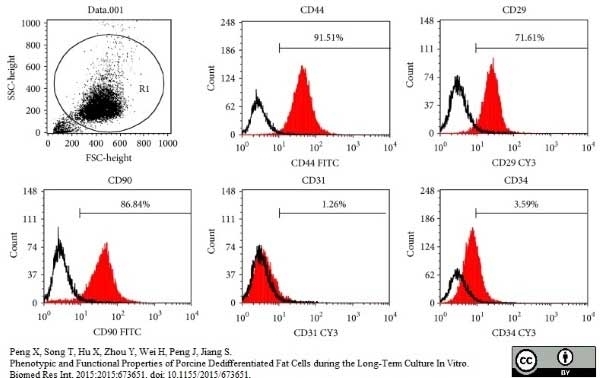
Peng, X. et al. (2015) Phenotypic and Functional Properties of Porcine Dedifferentiated Fat Cells during the Long-Term Culture In Vitro.
Biomed Res Int. 2015: 673651. -
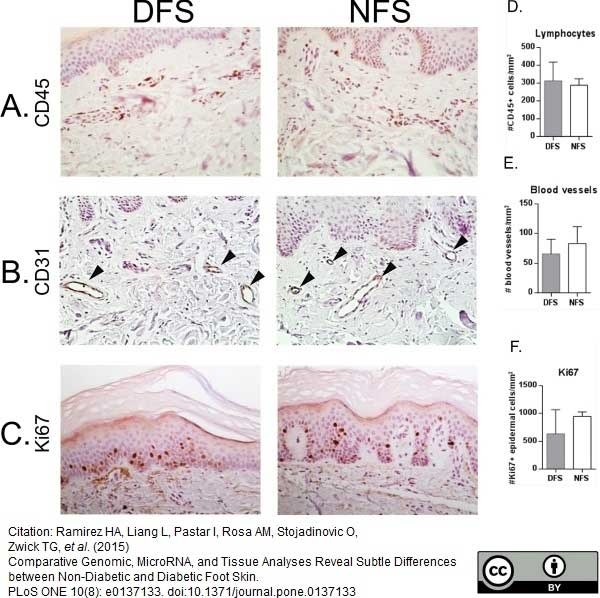
Ramirez, H.A. et al. (2015) Comparative Genomic, MicroRNA, and Tissue Analyses Reveal Subtle Differences between Non-Diabetic and Diabetic Foot Skin.
PLoS One. 10 (8): e0137133. -
Balaoing, L.R. et al. (2015) Laminin Peptide-Immobilized Hydrogels Modulate Valve Endothelial Cell Hemostatic Regulation.
PLoS One. 10 (6): e0130749. -
Barsotti, M.C. et al. (2015) Oligonucleotide biofunctionalization enhances endothelial progenitor cell adhesion on cobalt/chromium stents.
J Biomed Mater Res A. 103 (10): 3284-92. -
Zhang, Q. et al. (2015) Engineering vascularized soft tissue flaps in an animal model using human adipose-derived stem cells and VEGF+PLGA/PEG microspheres on a collagen-chitosan scaffold with a flow-through vascular pedicle.
Biomaterials. 73: 198-213. -
Puperi, D.S. et al. (2015) 3-Dimensional spatially organized PEG-based hydrogels for an aortic valve co-culture model.
Biomaterials. 67: 354-64. -
Ramm, R. et al. (2016) Decellularized GGTA1-KO pig heart valves do not bind preformed human xenoantibodies.
Basic Res Cardiol. 111 (4): 39. -
Leitão, A.F. et al. (2016) A Novel Small-Caliber Bacterial Cellulose Vascular Prosthesis: Production, Characterization, and Preliminary In Vivo Testing.
Macromol Biosci. 16 (1): 139-50. -
Chen, P. et al. (2017) Altered expression of eNOS, prostacyclin synthase, prostaglandin G/H synthase, and thromboxane synthase in porcine aortic endothelial cells after exposure to human serum-relevance to xenotransplantation.
Cell Biol Int. 41 (7): 798-808. -
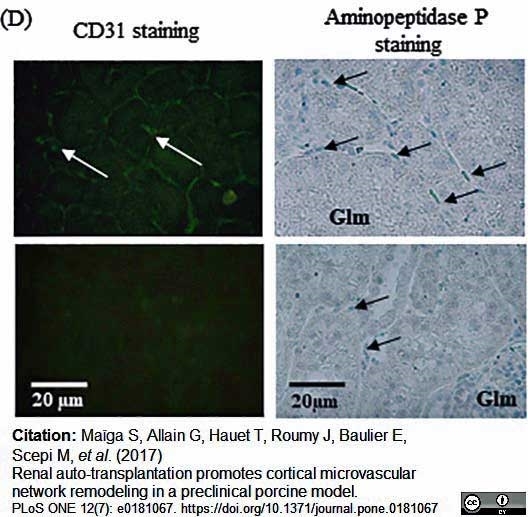
Maïga, S. et al. (2017) Renal auto-transplantation promotes cortical microvascular network remodeling in a preclinical porcine model.
PLoS One. 12 (7): e0181067. -
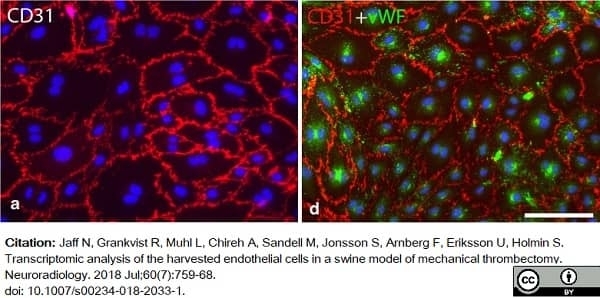
Jaff, N. et al. (2018) Transcriptomic analysis of the harvested endothelial cells in a swine model of mechanical thrombectomy.
Neuroradiology. 60 (7): 759-68. -
Strbo, N. et al. (2019) Single cell analyses reveal specific distribution of anti-bacterial molecule Perforin-2 in human skin and its modulation by wounding and Staphylococcus aureus infection.
Exp Dermatol. 28 (3): 225-32. -
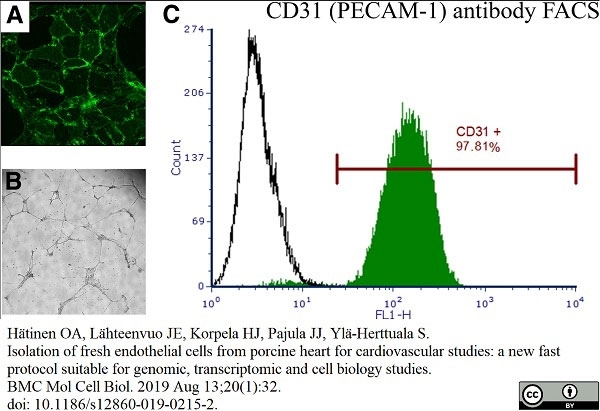
Hätinen, O.A. et al. (2019) Isolation of fresh endothelial cells from porcine heart for cardiovascular studies: a new fast protocol suitable for genomic, transcriptomic and cell biology studies.
BMC Mol Cell Biol. 20 (1): 32. -
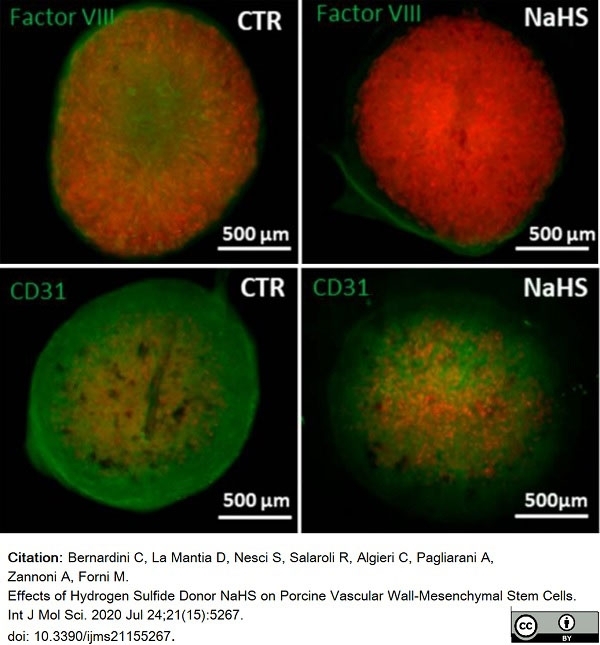
Bernardini, C. et al. (2020) Effects of Hydrogen Sulfide Donor NaHS on Porcine Vascular Wall-Mesenchymal Stem Cells.
Int J Mol Sci. 21(15):5267. -
Zhu, H. et al. (2022) Production of cultured meat from pig muscle stem cells.
Biomaterials. 287: 121650. -
Arenal, Á. et al. (2022) Effects of Cardiac Stem Cell on Postinfarction Arrhythmogenic Substrate.
Int J Mol Sci. 23 (24): 16211. -
Burdorf, L. et al. (2023) Expression of human thrombomodulin by GalTKO.hCD46 pigs modulates coagulation cascade activation by endothelial cells and during ex vivo lung perfusion with human blood.
Xenotransplantation. : e12828. -
Bernardini, C. et al. (2023) Isolation of Vascular Wall Mesenchymal Stem Cells from the Thoracic Aorta of Adult Göttingen Minipigs: A New Protocol for the Simultaneous Endothelial Cell Collection.
Animals (Basel). 13 (16): 2601.
Further Reading
-
Piriou-Guzylack, L. (2008) Membrane markers of the immune cells in swine: an update.
Vet Res. 39: 54. -
Rayat, G.R. et al. (2016) First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes - Chapter 3: Porcine islet product manufacturing and release testing criteria.
Xenotransplantation. 23 (1): 38-45.
- Synonyms
- PECAM-1
- RRID
- AB_2252096
- UniProt
- Q95242
- Entrez Gene
- PECAM1
- GO Terms
- GO:0007155 cell adhesion
- GO:0016021 integral to membrane
- GO:0030054 cell junction
MCA1746PET
MCA1746PE
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Pig ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up