Omalizumab antibody | AbD20669_hIgG1




Human anti Omalizumab
- Product Type
- Monoclonal Antibody
- Clone
- AbD20669_hIgG1
- Isotype
- IgG1
- Specificity
- Omalizumab
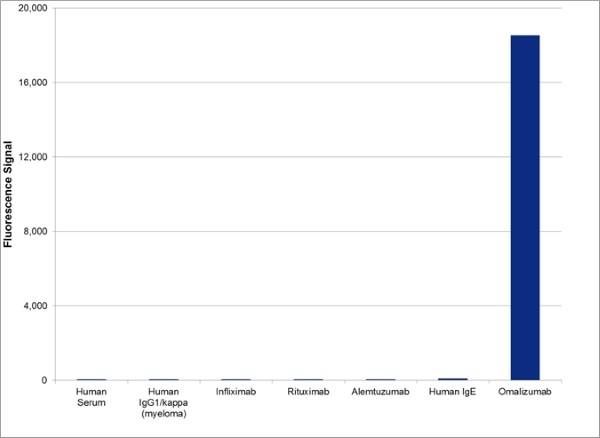
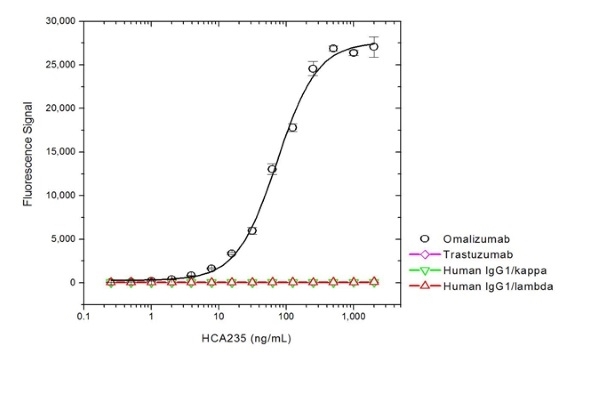
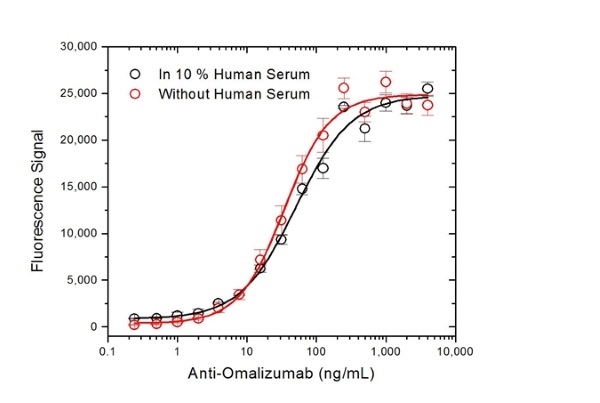
| Human Anti-Omalizumab Antibody, clone AbD20669_hIgG1 is a paratope specific, anti-idiotypic antibody that binds specifically to omalizumab but not to free IgE or the drug/immunoglobulin complex. The antibody can be used to measure the levels of omalizumab and biosimilar products in bioanalytical assays. Clone AbD20669_hIgG1 can be used to develop a pharmacokinetic (PK) bridging assay to measure free drug. This antibody, HRP-conjugated and in full immunoglobulin (Ig) format, is recommended as the detection antibody, paired with the antibody in monovalent Fab format, clone AbD20669 (HCA236), as the capture antibody. The antibody in Ig format can also be used to develop and calibrate immune response assays to measure the anti-drug antibody (ADA) response in patient sera. Omalizumab (brand name Xolair) is a recombinant DNA-derived humanized IgG1 kappa monoclonal antibody used in the treatment of patients with moderate or severe asthma who have demonstrated a positive allergy skin test and whose symptoms are not controlled by inhaled corticosteroids. Allergic asthma is mediated by IgE released by B cells in response to allergen. Circulating IgE binds to the high-affinity IgE Fc receptor (FcεRI) expressed on basophils and mast cells, triggering the release of histamine, leukotrienes and other mediators associated with the pathophysiology of asthma. Omalizumab is directed against the Fc region of human immunoglobulin E (IgE). By binding to circulating IgE at the site of FcεRI binding, this therapeutic antibody prevents the interaction of IgE with its receptor thus limiting mediator release. Treatment with omalizumab has also been demonstrated to reduce the expression of FcεRI on mast cells and basophils, providing additional clinical benefit. View a summary of all Anti-Omalizumab Antibodies |
|
- Product Form
- Human IgG1 antibody (lambda light chain) selected from the HuCAL phage display library and expressed in a human cell line. This antibody is supplied as a liquid.
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A
- Source
- HKB-11
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% Sodium Azide (NaN3)
- Immunogen
- Omalizumab
- Affinity
- HCA235G: The intrinsic affinity of the monovalent form of this antibody is KD = 1.1 nM as measured by real time, label free molecular interaction analysis on immobilized omalizumab.
- HCA235: The intrinsic affinity of the monovalent form this antibody is KD = 1.1 nM as measured by real time, label free molecular interaction analysis on immobilized omalizumab.
- Approx. Protein Concentrations
- HCA235G: IgG concentration 1.0 mg/ml
- HCA235: IgG concentration 0.5 mg/ml
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
- Acknowledgements
- This product and/or its use is covered by claims of U.S. patents, and/or pending U.S. and non-U.S. patent applications owned by or under license to Bio-Rad Laboratories, Inc. See bio-rad.com/en-us/trademarks for details.
Xolair is a trademark of Novartis. - Licensed Use
- HCA235G: For in vitro research purposes and for commercial applications for the provision of in vitro testing services to support preclinical and clinical drug development. Any re-sale in any form or any other commercial application needs a written agreement with Bio-Rad.
- HCA235: For in vitro research purposes and for commercial applications for the provision in vitro testing services to support preclinical and clinical drug development. Any re-sale in any form or any other commercial application needs a written agreement with Bio-Rad.
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA |
- Technical Advice
- Recommended protocols and further information about HuCAL recombinant antibody technology can be found in the HuCAL Antibodies Technical Manual.
- ELISA
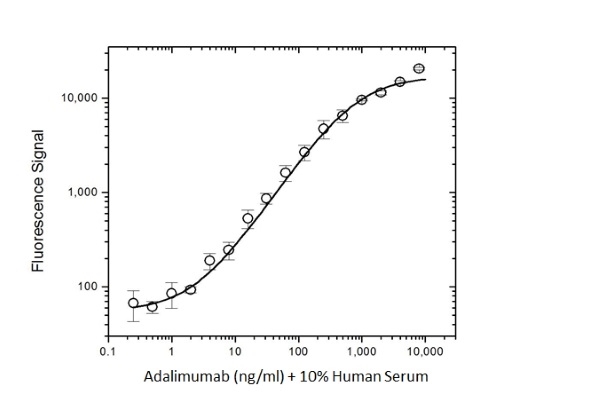
- This product may be used as bridging reagent in ADA assay development or, following HRP-conjugation, as a detection reagent in PK assay development together with HCA236 as the capture reagent.
Protocol: PK bridging ELISA to measure free drug
Protocol: ADA bridging ELISA.
References for Omalizumab antibody
-
Kashiwagi, N. et al. (2017) Method for measuring anti-drug antibody
US Patent application US20170315118A1
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
Always be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up