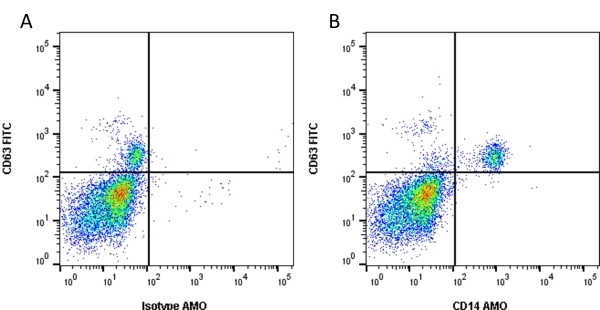
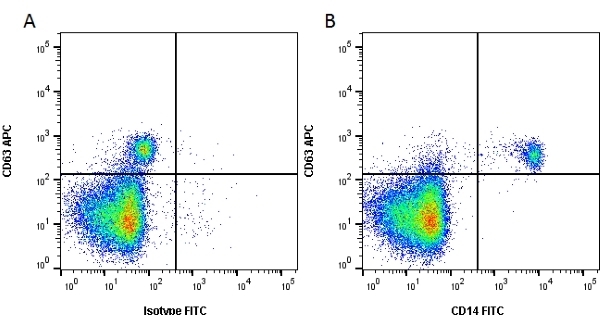
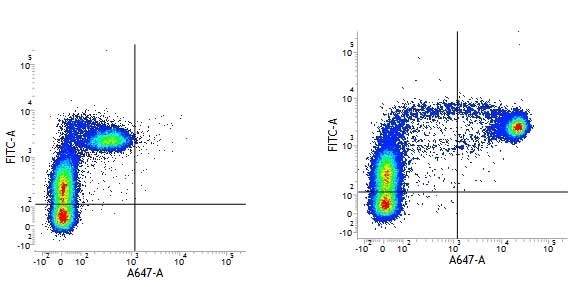
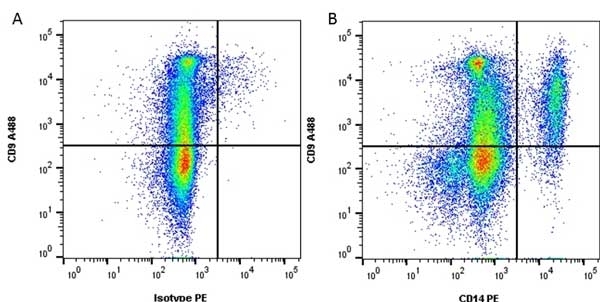
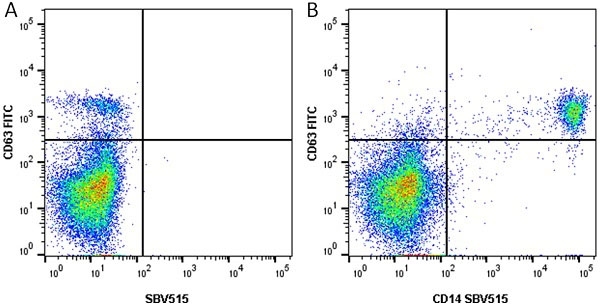
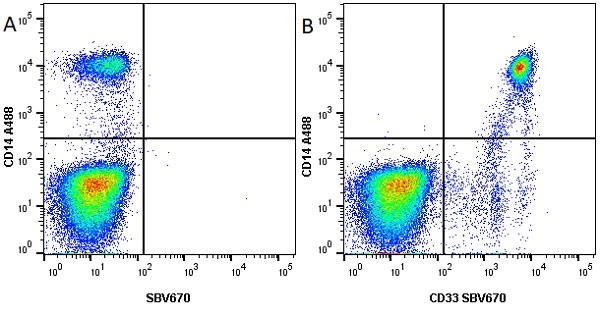
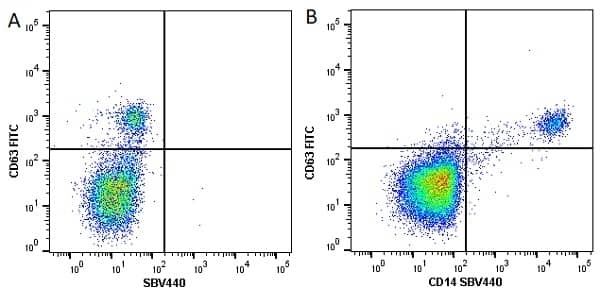
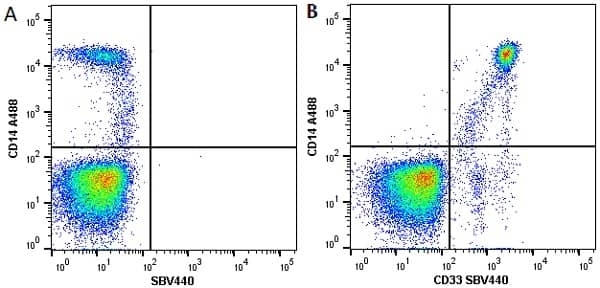
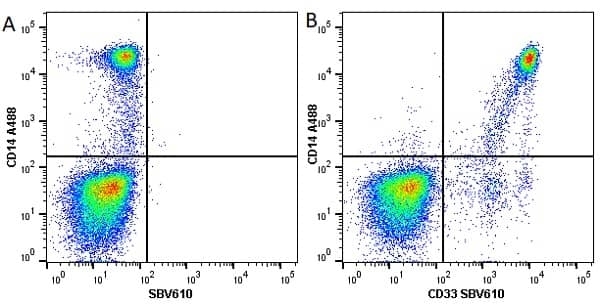
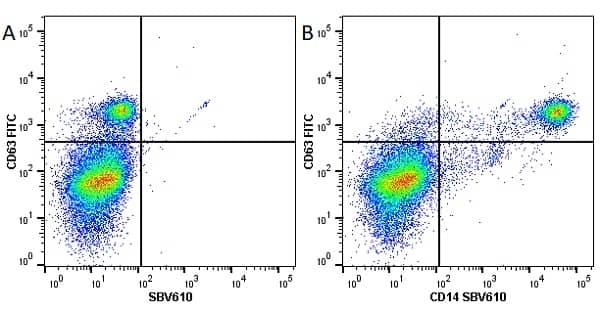
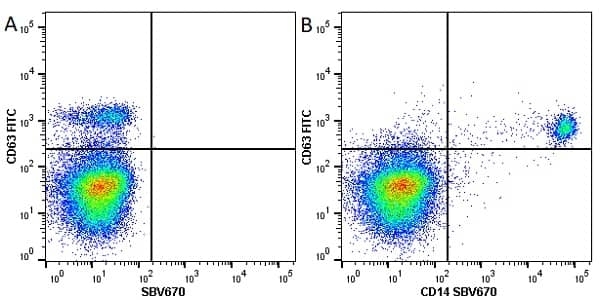
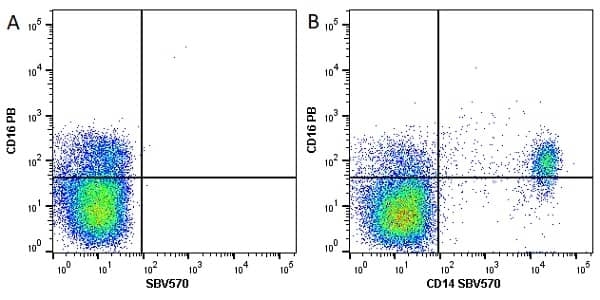
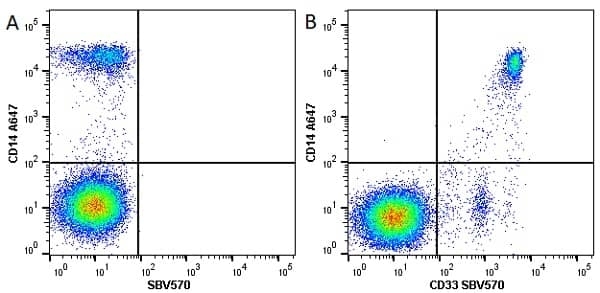
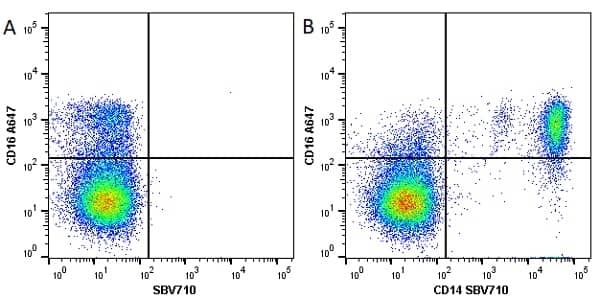
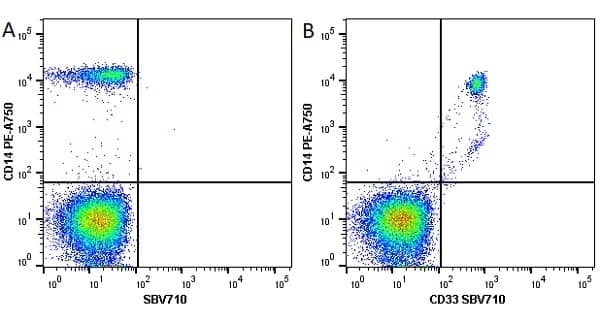
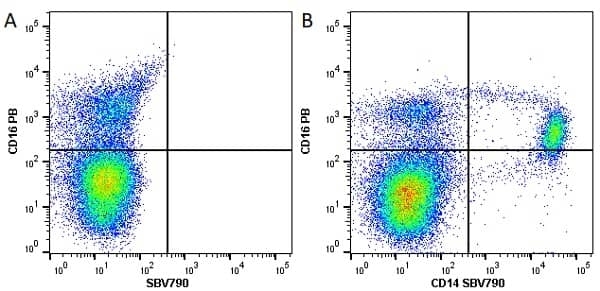
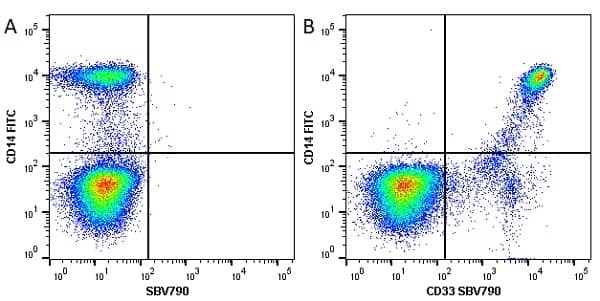
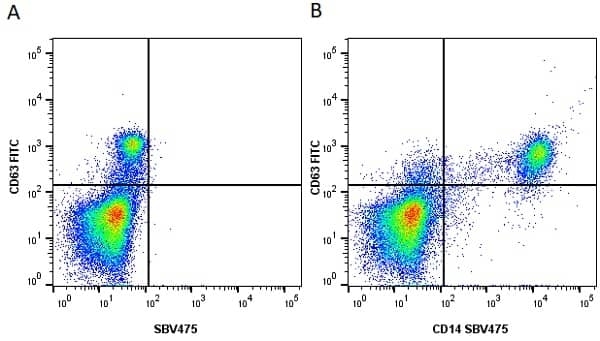
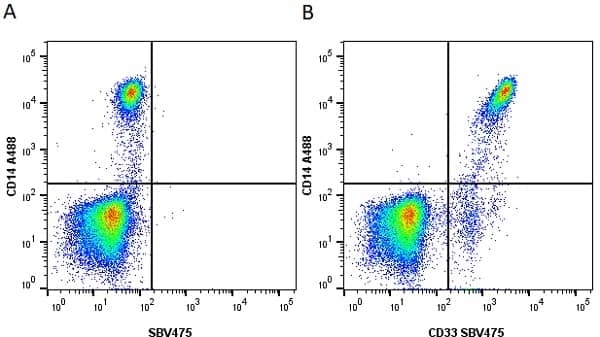
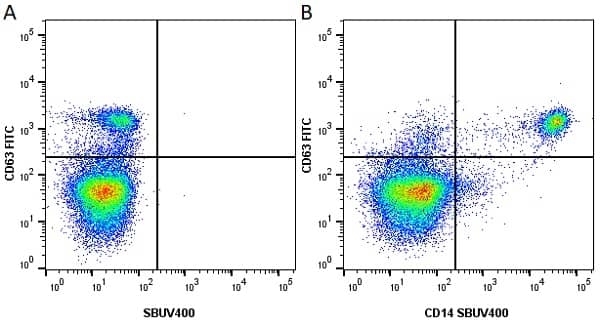
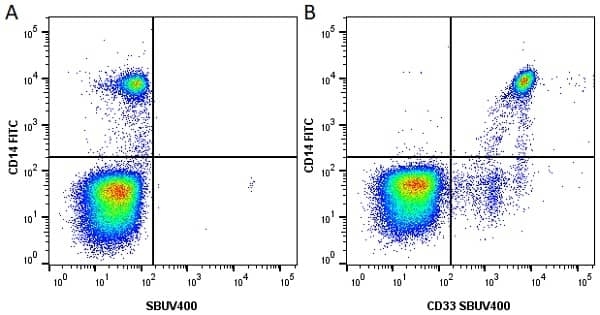
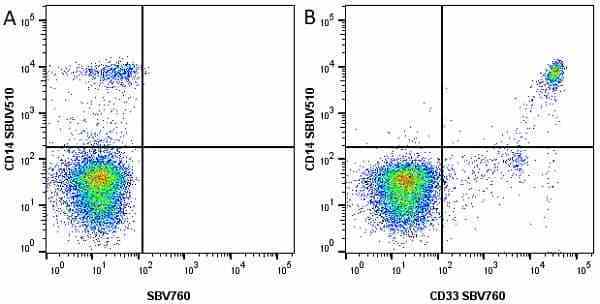
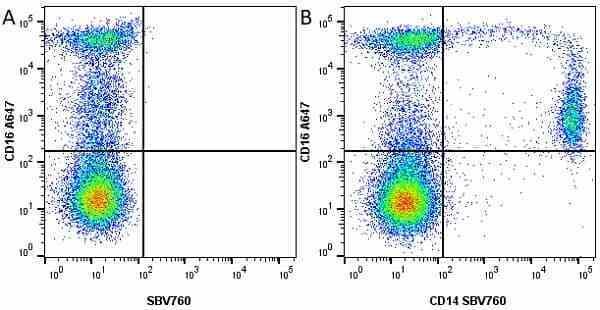
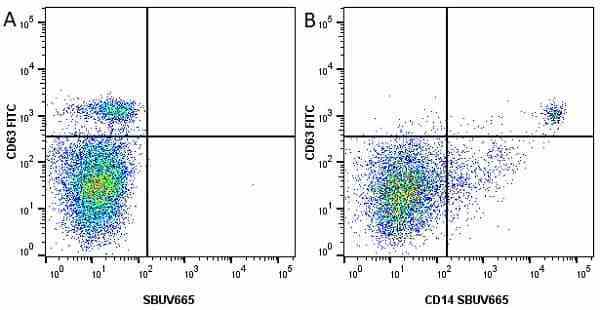
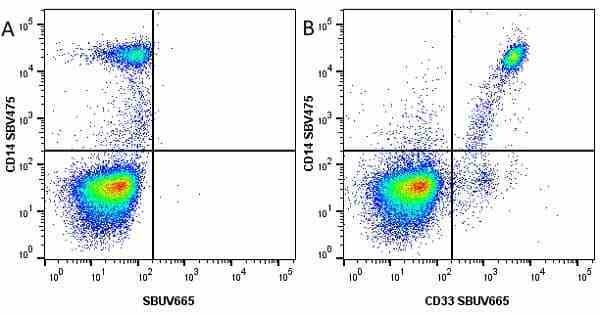
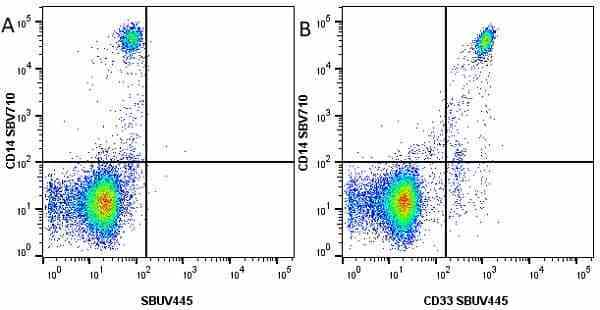
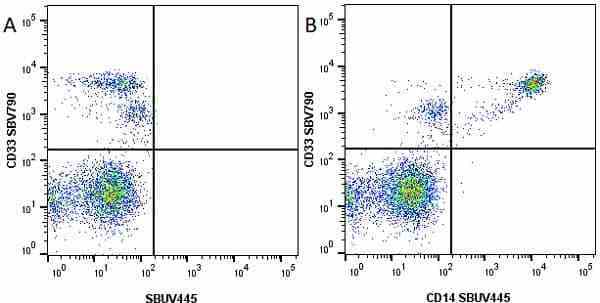
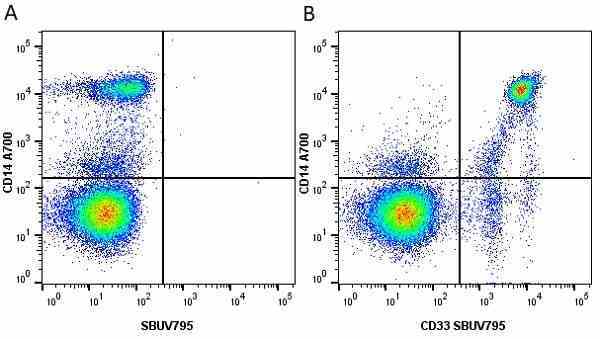
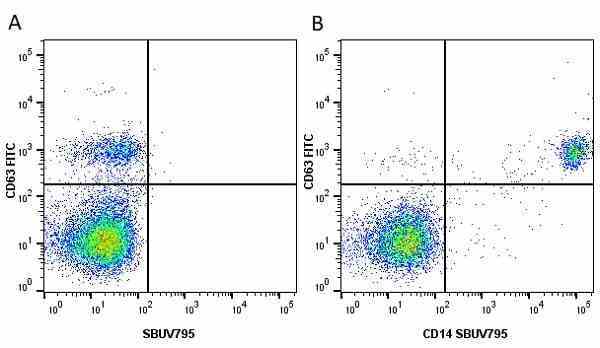
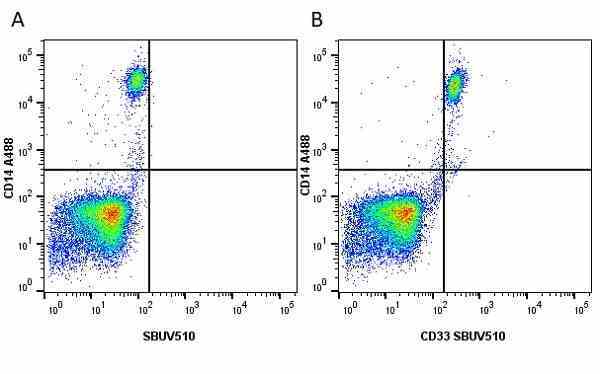
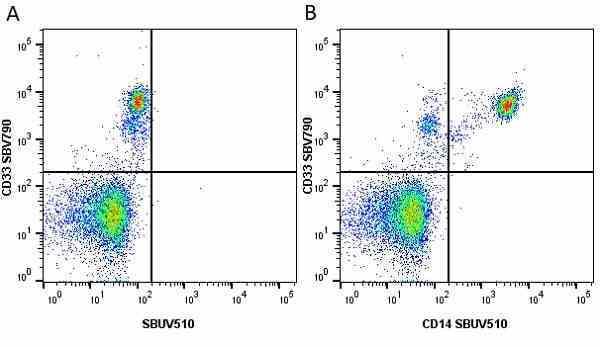
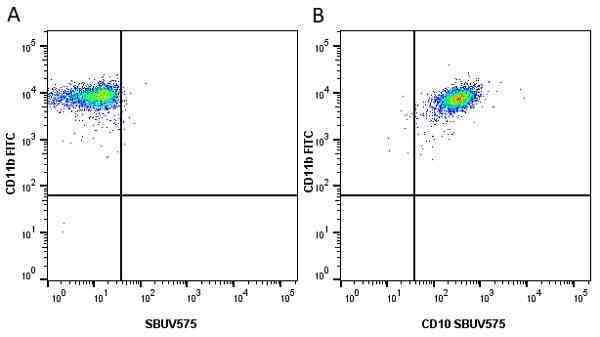
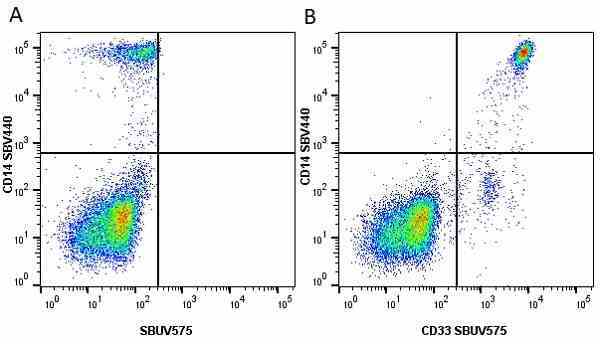
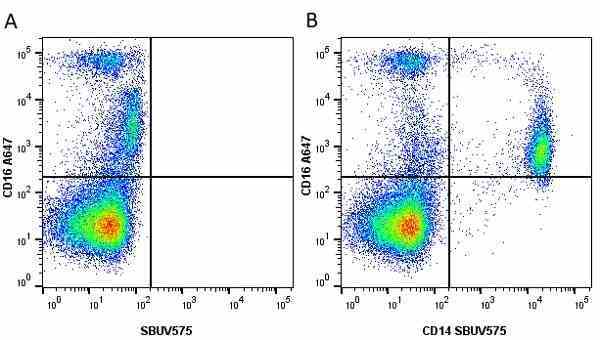
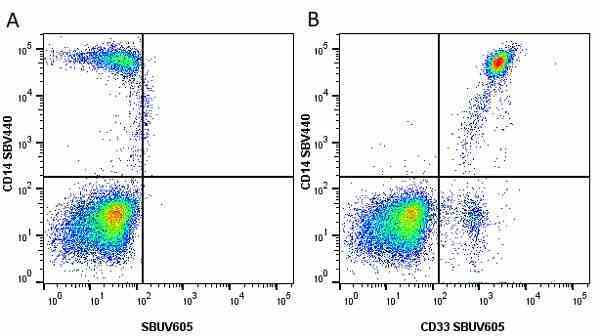
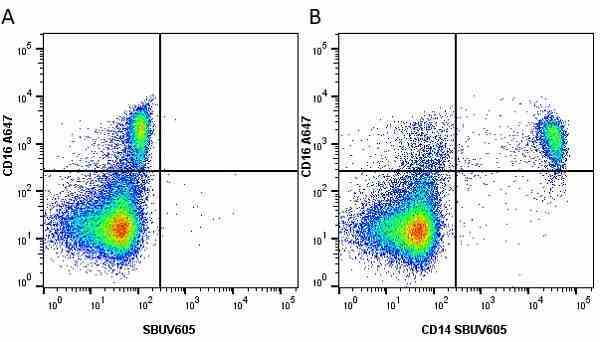
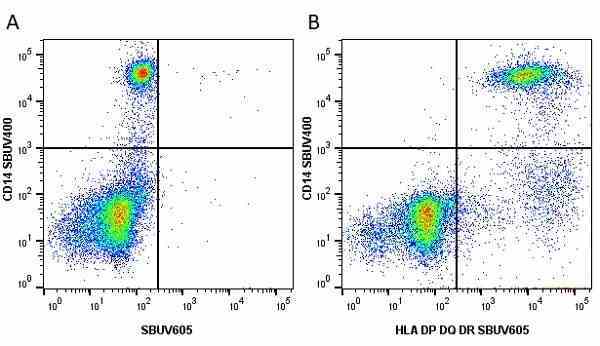
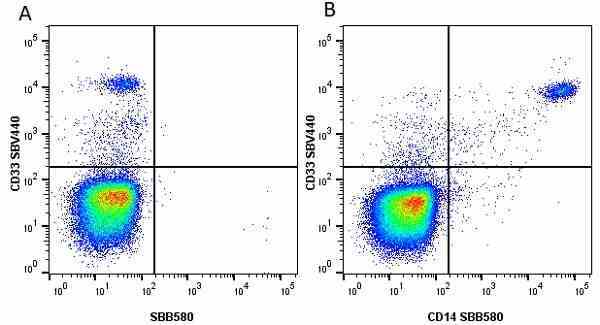
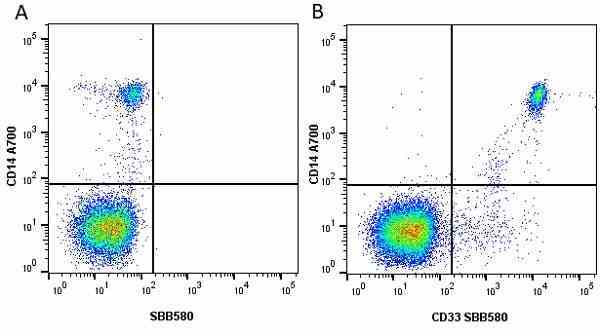
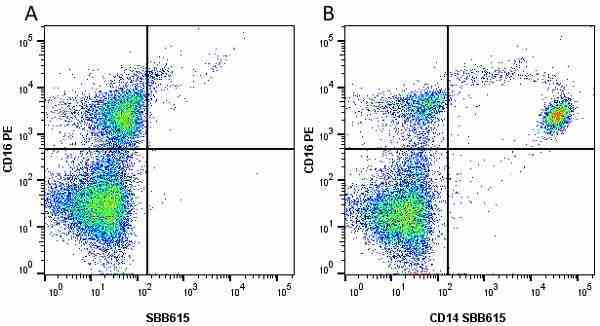
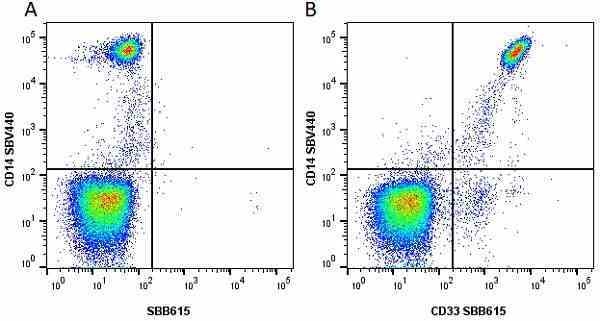
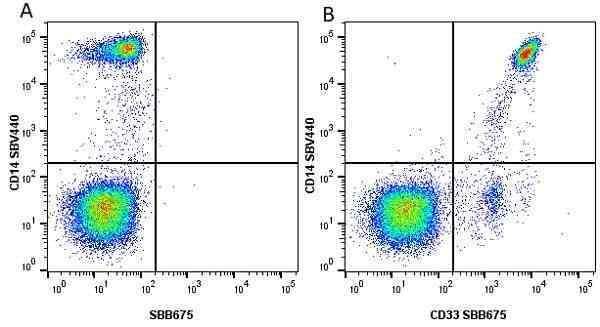
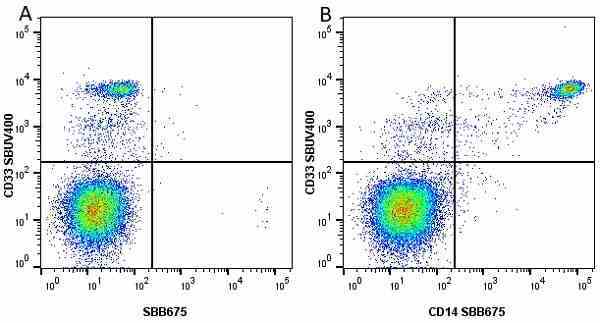
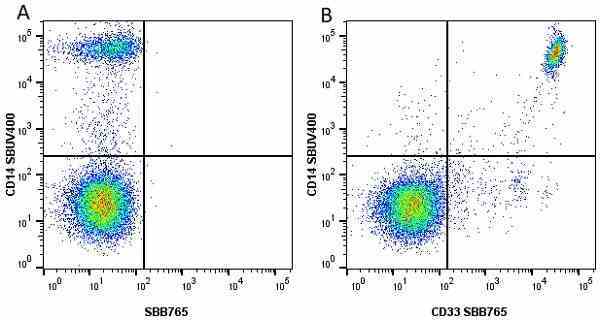
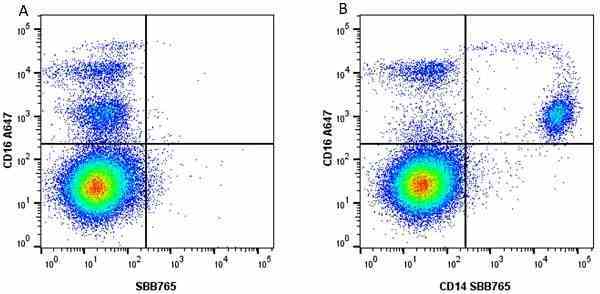
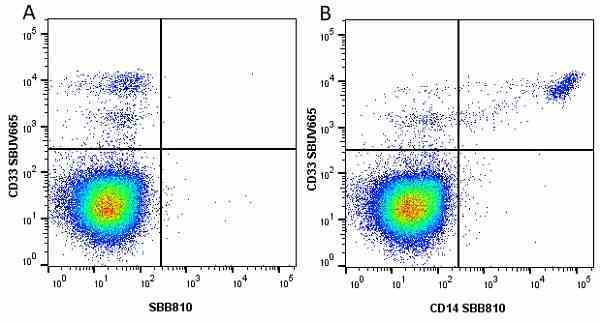
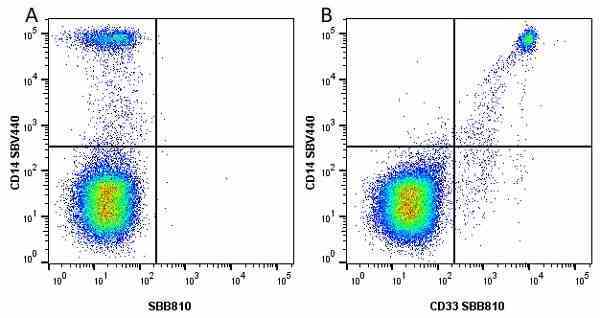
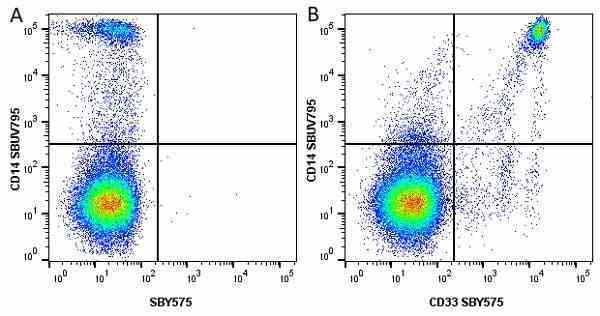
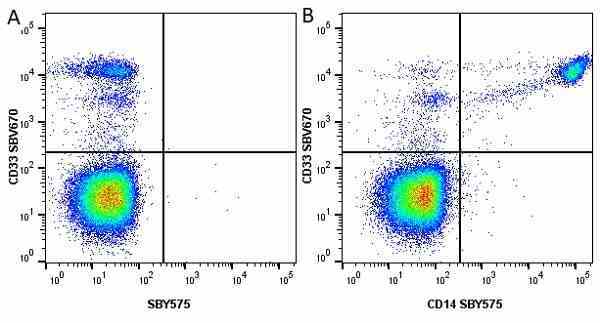
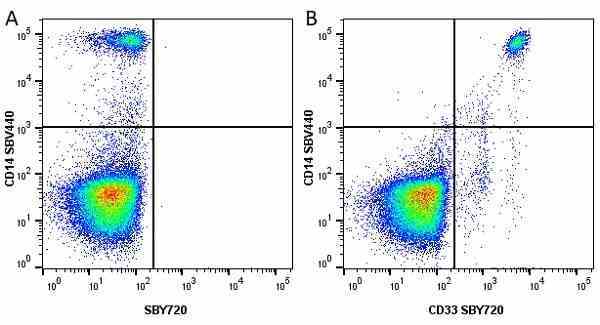
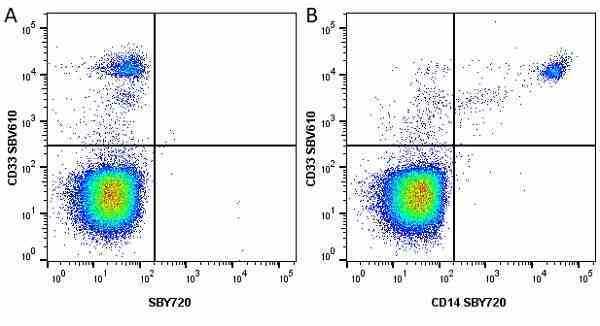
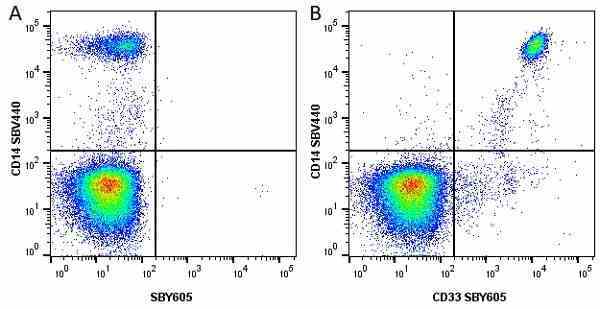
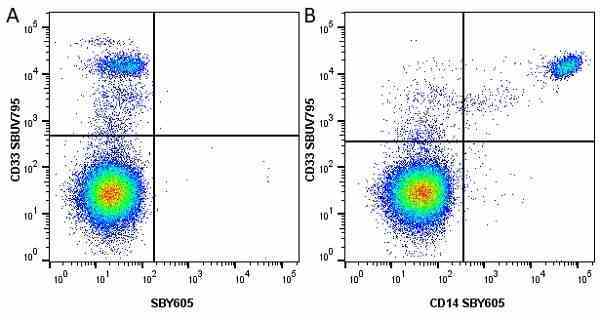
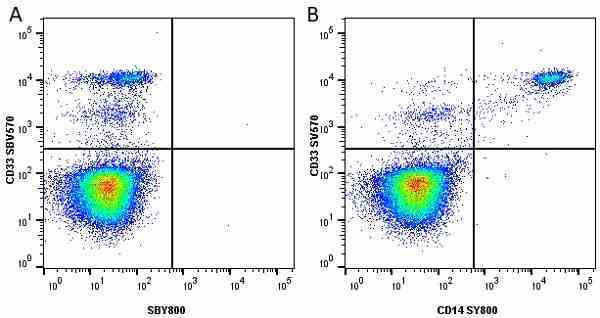
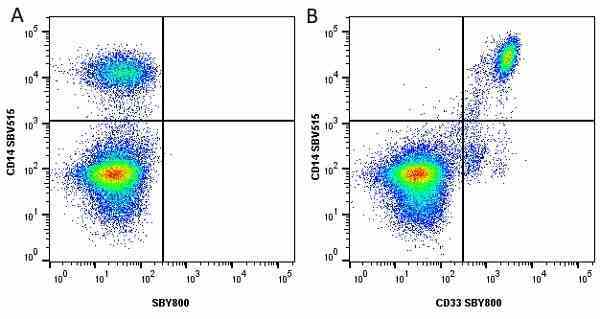
CD14 antibody | TÜK4







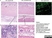
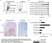
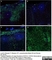
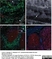





























































Mouse anti Human CD14:StarBright Violet 760
- Product Type
- Monoclonal Antibody
- Clone
- TÜK4
- Isotype
- IgG2a
- Specificity
- CD14
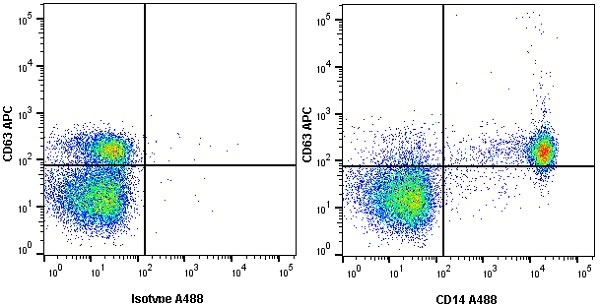
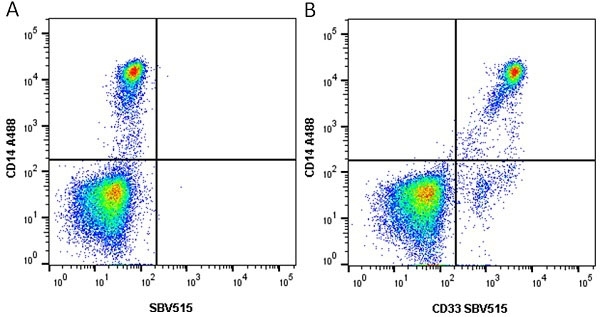
| Mouse anti Human CD14 antibody, clone TÜK4 recognizes the human CD14 cell surface antigen. CD14 is a ~55 kDa glycoprotein that contains multiple leucine-rich repeats. It is anchored to the cell membrane via a glycosylphosphatidylinositol (GPI) linkage (Simmons et al. 1989), a soluble form of CD14 also exists (Bazil et al. 1986). CD14 is strongly expressed on the surface of monocytes and macrophages but has also been shown to be expressed on the surface of non-myeloid cells (Jersmann 2005). CD14 functions as a pattern recognition receptor (Pugin et al. 1994, Dziarski et al. 1998) in innate immunity for a variety of ligands, in particular for the LPS (endotoxin) of Gram-negative bacteria. Mouse anti human CD14 antibody, clone TÜK4 has been shown to block SDF-induced chemotaxis of U937 cells in a dose –dependent manner (Yang et al. 2003). Use of the anti-human CD14 antibody, Low Endotoxin format is recommended for this purpose. |
- Target Species
- Human
- Species Cross-Reactivity
-
Target Species Cross Reactivity Dog Goat Cat Rabbit Mink Bovine Pig Sheep Cynomolgus monkey Llama - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG conjugated to StarBright Violet 760 - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
1% bovine serum albumin
0.1% Pluronic F68
0.1% PEG 3350
0.05% Tween 20 - Max Ex/Em
-
Fluorophore Excitation Max (nm) Emission Max (nm) StarBright Violet 760 403 754 - Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
- Acknowledgements
- This product is covered by U.S. Patent No. 10,150,841 and related U.S. and foreign counterparts
DO NOT FREEZE.
This product should be stored undiluted.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | Neat |
- Flow Cytometry
- Use 5μl of the suggested working dilution to label 106 cells in 100μl. Best practices suggest a 5 minutes centrifugation at 6,000g prior to sample application.
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Human Seroblock | BUF070A | F | 50 Test | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Human Seroblock | ||||||
| Human Seroblock | BUF070B | F | 200 Test | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Human Seroblock | ||||||
References for CD14 antibody
-
Jacobsen, C.N. et al. (1993) Reactivities of 20 anti-human monoclonal antibodies with leucocytes from ten different animal species.
Vet Immunol Immunopathol. 39 (4): 461-6. -
Gupta, V.K. et al. (1996) Identification of the sheep homologue of the monocyte cell surface molecule--CD14.
Vet Immunol Immunopathol. 51 (1-2): 89-99. -
Sopp, P. & Howard, C.J. (1997) Cross-reactivity of monoclonal antibodies to defined human leucocyte differentiation antigens with bovine cells.
Vet Immunol Immunopathol. 56 (1-2): 11-25. -
Werling, D. et al. (1998) Analysis of the phenotype and phagocytic activity of monocytes/macrophages from cattle infected with the bovine leukaemia virus.
Vet Immunol Immunopathol. 62 (3): 185-95. -
Weiss, D.J. (2001) Evaluation of proliferative disorders in canine bone marrow by use of flow cytometric scatter plots and monoclonal antibodies.
Vet Pathol. 38: 512-8. -
Bryan, S.A. et al. (2002) Responses of leukocytes to chemokines in whole blood and their antagonism by novel CC-chemokine receptor 3 antagonists.
Am J Respir Crit Care Med. 165: 1602-9. -
Yang, H. et al. (2003) Antibody to CD14 like CXCR4-specific antibody 12G5 could inhibit CXCR4-dependent chemotaxis and HIV Env-mediated cell fusion.
Immunol Lett. 88 (1): 27-30. -
Schenk, M. et al. (2005) Macrophages expressing triggering receptor expressed on myeloid cells-1 are underrepresented in the human intestine.
J Immunol. 174 (1): 517-24.
View The Latest Product References
-
Fulton, B.E. Jr. et al. (2006) Dissemination of bovine leukemia virus-infected cells from a newly infected sheep lymph node.
J Virol. 80: 7873-84. -
Willett, B.J. et al. (2007) Probing the interaction between feline immunodeficiency virus and CD134 by using the novel monoclonal antibody 7D6 and the CD134 (Ox40) ligand.
J Virol. 81: 9665-79. -
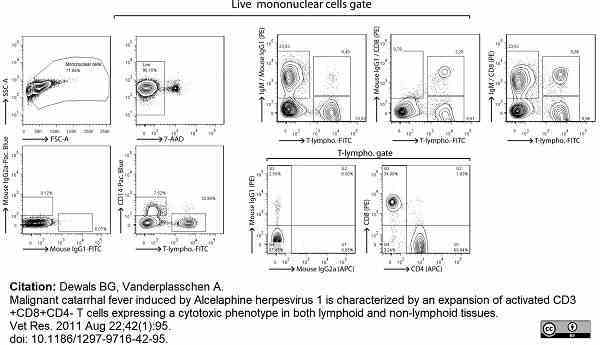
Dewals, B.G. & Vanderplasschen, A. (2011) Malignant catarrhal fever induced by Alcelaphine herpesvirus 1 is characterized by an expansion of activated CD3+CD8+CD4- T cells expressing a cytotoxic phenotype in both lymphoid and non-lymphoid tissues.
Vet Res. 42 (1): 95. -
Dalli J et al. (2008) Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles.
Blood. 112 (6): 2512-9. -
Martel, C.J. & Aasted, B. (2009) Characterization of antibodies against ferret immunoglobulins, cytokines and CD markers.
Vet Immunol Immunopathol. 132:109-15. -
Lybeck, K.R. et al. (2009) Neutralization of interleukin-10 from CD14(+) monocytes enhances gamma interferon production in peripheral blood mononuclear cells from Mycobacterium avium subsp. paratuberculosis-infected goats.
Clin Vaccine Immunol. 16 (7): 1003-11. -
Ferret-Bernard, S. et al. (2010) Cellular and molecular mechanisms underlying the strong neonatal IL-12 response of lamb mesenteric lymph node cells to R-848.
PLoS One. 5: e13705. -
Xiong, W. et al. (2010) Human Flt3L generates dendritic cells from canine peripheral blood precursors: implications for a dog glioma clinical trial.
PLoS One. 5: e11074. -
Kallapur, S.G. et al. (2011) Pulmonary and systemic inflammatory responses to intra-amniotic IL-1α in fetal sheep.
Am J Physiol Lung Cell Mol Physiol. 301 (3): L285-95. -
Gelain, M.E. et al. (2014) CD44 in canine leukemia: analysis of mRNA and protein expression in peripheral blood.
Vet Immunol Immunopathol. 159 (1-2): 91-6. -
Schaut, R.G. et al. (2015) Bovine viral diarrhea virus type 2 in vivo infection modulates TLR4 responsiveness in differentiated myeloid cells which is associated with decreased MyD88 expression.
Virus Res. 208: 44-55. -
Novacco, M. et al. (2016) Prognostic factors in canine acute leukaemias: a retrospective study.
Vet Comp Oncol. 14 (4): 409-16. -
Gibson, A.J. et al. (2016) Differential macrophage function in Brown Swiss and Holstein Friesian cattle.
Vet Immunol Immunopathol. 181: 15-23. -
Krueger, L.A. et al. (2016) Gamma delta T cells are early responders to Mycobacterium avium ssp. paratuberculosis in colostrum-replete Holstein calves.
J Dairy Sci. 99 (11): 9040-50. -
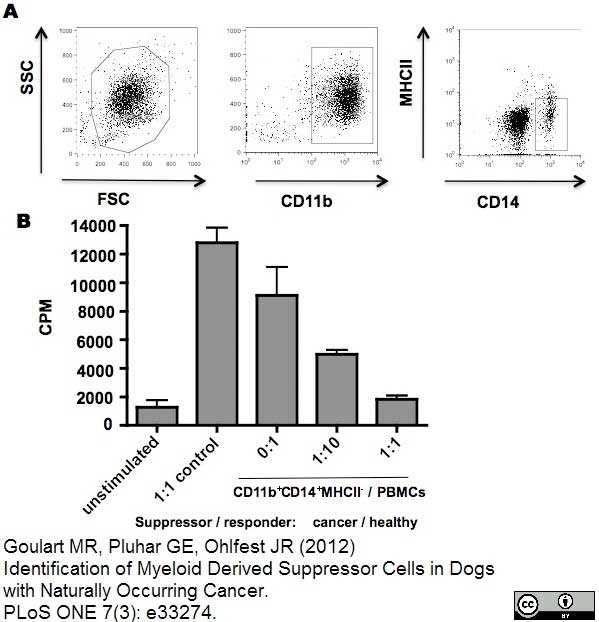
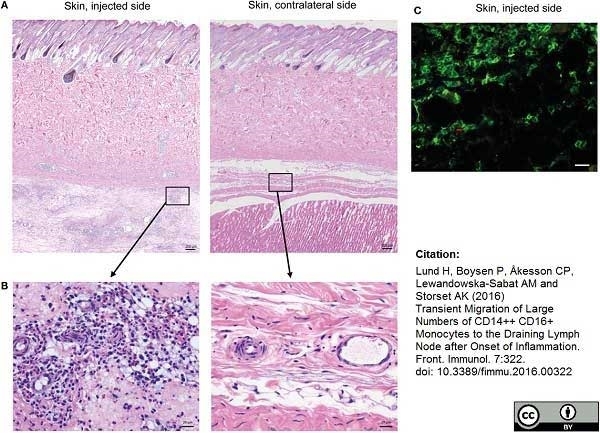
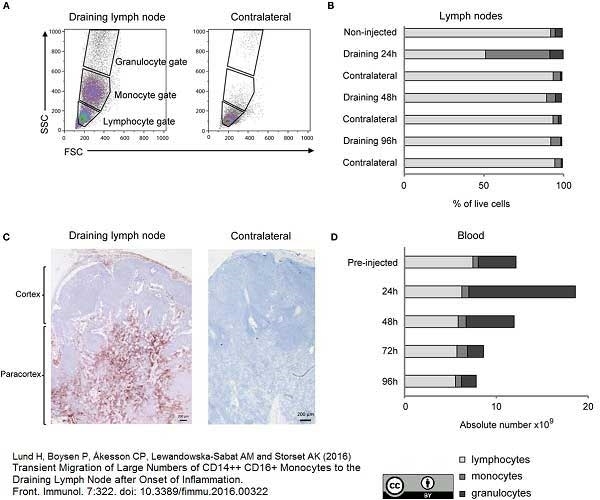
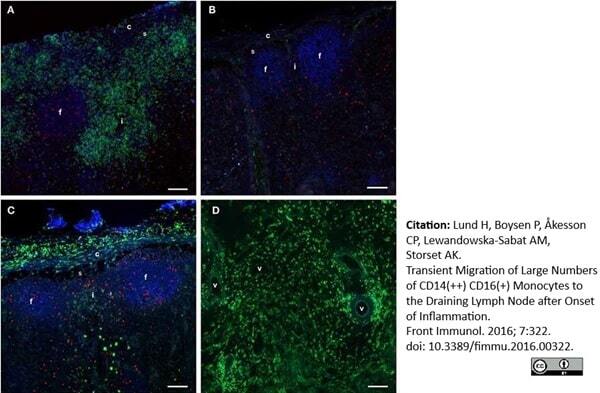
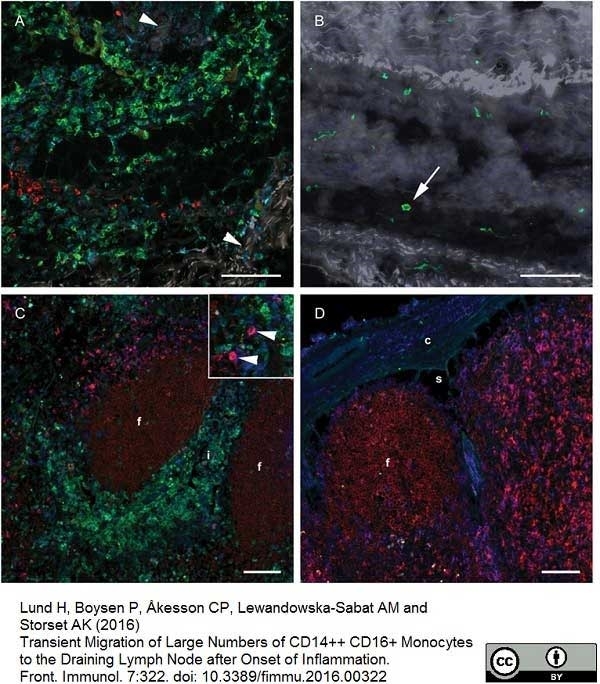
Lund, H. et al. (2016) Transient Migration of Large Numbers of CD14(++) CD16(+) Monocytes to the Draining Lymph Node after Onset of Inflammation.
Front Immunol. 7: 322. -
Westover, A.J. et al. (2016) An Immunomodulatory Device Improves Insulin Resistance in Obese Porcine Model of Metabolic Syndrome.
J Diabetes Res. 2016: 3486727. -
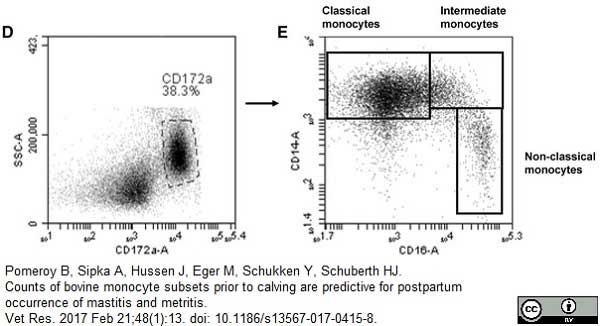
Pomeroy, B. et al. (2017) Counts of bovine monocyte subsets prior to calving are predictive for postpartum occurrence of mastitis and metritis.
Vet Res. 48 (1): 13. -
Martini, V. et al. (2018) Flow cytometry for feline lymphoma: a retrospective study regarding pre-analytical factors possibly affecting the quality of samples.
J Feline Med Surg. 20 (6): 494-501. -
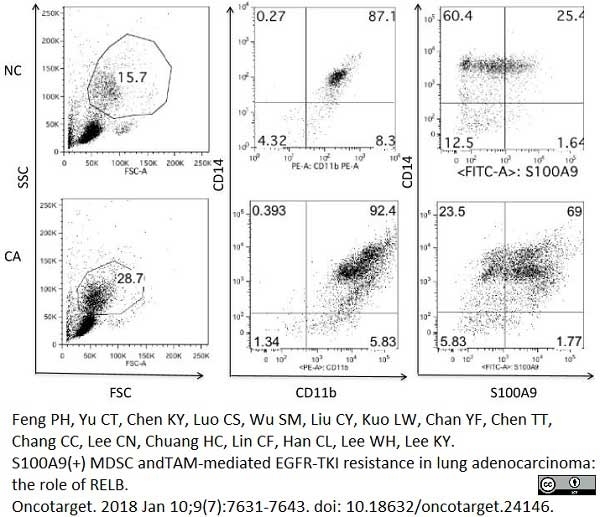
Feng, P.H. et al. (2018) S100A9+ MDSC and TAM-mediated EGFR-TKI resistance in lung adenocarcinoma: the role of RELB.
Oncotarget. 9 (7): 7631-43. -
Higgins, J.L. et al. (2018) Cell mediated immune response in goats after experimental challenge with the virulent Brucella melitensis strain 16M and the reduced virulence strain Rev. 1.
Vet Immunol Immunopathol. 202: 74-84. -
Lessard, M. et al. (2018) Piglet weight gain during the first two weeks of lactation influences the immune system development.
Vet Immunol Immunopathol. 206: 25-34. -
Moncada-Saucedo, N.K. et al. (2019) A Bioactive Cartilage Graft of IGF1-Transduced Adipose Mesenchymal Stem Cells Embedded in an Alginate/Bovine Cartilage Matrix Tridimensional Scaffold.
Stem Cells Int. 2019: 9792369. -
Kolar, Q.K. et al. (2020) Anatomical distribution of respiratory tract leukocyte cell subsets in neonatal calves.
Vet Immunol Immunopathol. 227: 110090. -
Risalde, M.A. et al. (2020) BVDV permissiveness and lack of expression of co-stimulatory molecules on PBMCs from calves pre-infected with BVDV.
Comp Immunol Microbiol Infect Dis. 68: 101388. -
Muñoz-Silvestre, A. et al. (2020) Pathogenesis of Intradermal Staphylococcal Infections: Rabbit Experimental Approach to Natural Staphylococcus aureus Skin Infections.
Am J Pathol. 190 (6): 1188-210. -
Sipka, A.S. et al. (2020) The effect of ex vivo. lipopolysaccharide stimulation and nutrient availability on transition cow innate immune cell AKT/mTOR pathway responsiveness.
J Dairy Sci. 103 (2): 1956-1968. -
Mas, A. et al. (2020) A further investigation of the leishmaniosis outbreak in Madrid (Spain): low-infectivity phenotype of the Leishmania infantum BOS1FL1 isolate to establish infection in canine cells.
Vet Immunol Immunopathol. 230: 110148. -
Schwarz, E.R. et al. (2020) Experimental Infection of Mid-Gestation Pregnant Female and Intact Male Sheep with Zika Virus.
Viruses. 12 (3): 291. -
Penadés, M. et al. (2020) Early deviations in performance, metabolic and immunological indicators affect stayability in rabbit females.
Animal. 14 (4): 780-9. -
Tuohy, J.L. et al. (2020) Immune dysregulation and osteosarcoma: Staphylococcus aureus. downregulates TGF-β and heightens the inflammatory signature in human and canine macrophages suppressed by osteosarcoma.
Vet Comp Oncol. 18 (1): 64-75. -
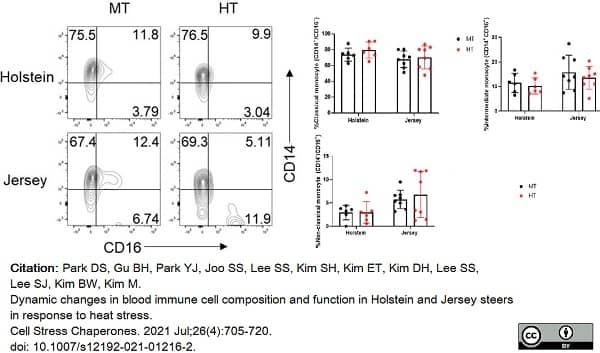
Park, D.S. et al. (2021) Dynamic changes in blood immune cell composition and function in Holstein and Jersey steers in response to heat stress.
Cell Stress Chaperones. 26 (4): 705-20. -
Grudzien, M. et al. (2021) A newly established canine NK-type cell line and its cytotoxic properties.
Vet Comp Oncol. 19 (3): 567-77. -
Jaensch, S.M. et al. (2022) Clinicopathologic and immunophenotypic features in dogs with presumptive large granular lymphocyte leukaemia.
Aust Vet J. 100 (11): 527-32. -
Riccardo, F. et al. (2022) Antigen mimicry as an effective strategy to induce CSPG4-targeted immunity in dogs with oral melanoma: a veterinary trial.
J Immunother Cancer. 10(5):e004007. -
Shiue, S.J. et al. (2022) Arthrospira Enhances Seroclearance in Patients with Chronic Hepatitis B Receiving Nucleos(t)ide Analogue through Modulation of TNF-α/IFN-γ Profile.
Nutrients. 14 (14): 2790. -
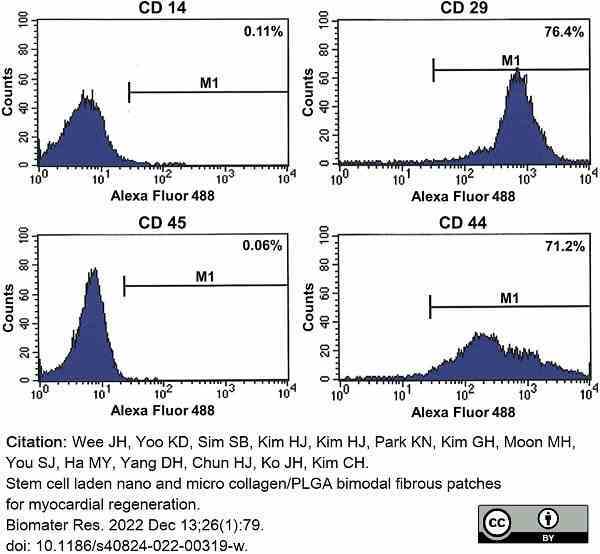
Wee, J.H. et al. (2022) Stem cell laden nano and micro collagen/PLGA bimodal fibrous patches for myocardial regeneration.
Biomater Res. 26 (1): 79. -
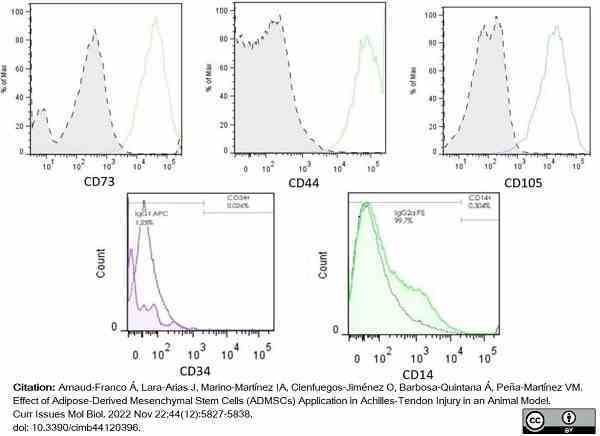
Arnaud-Franco, Á. et al. (2022) Effect of Adipose-Derived Mesenchymal Stem Cells (ADMSCs) Application in Achilles-Tendon Injury in an Animal Model.
Curr Issues Mol Biol. 44 (12): 5827-38. -
Ashwood, P. (2022) Preliminary Evidence of Differentially Induced Immune Responses by Microparticle-adsorbed LPS in Patients with Crohn's Disease.
J Cell Immunol. 4 (6): 211-218. -
Rotolo, A. et al. (2023) Unedited allogeneic iNKT cells show extended persistence in MHC-mismatched canine recipients.
Cell Rep Med. 4 (10): 101241. -
Rütgen, B.C. et al. (2022) Composition of lymphocyte subpopulations in normal and mildly reactive peripheral lymph nodes in cats.
J Feline Med Surg. 24 (2): 77-90. -
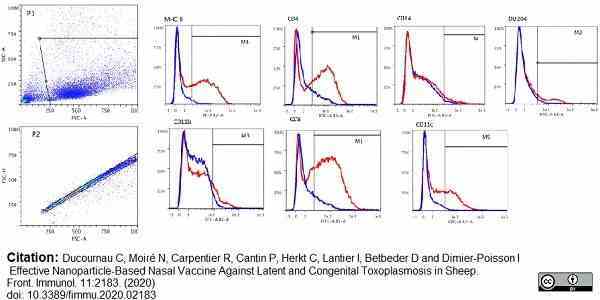
Ducournau, C. et al. (2020) Effective Nanoparticle-Based Nasal Vaccine Against Latent and Congenital Toxoplasmosis in Sheep
Front Immunol. 11:2183. -
Sheng, R. et al. (2023) Prognostic significance of CD25 expression in dogs with a noninvasive diagnosis of B-cell lymphoma treated with CHOP chemotherapy.
Vet Comp Oncol. 21 (1): 28-35. -
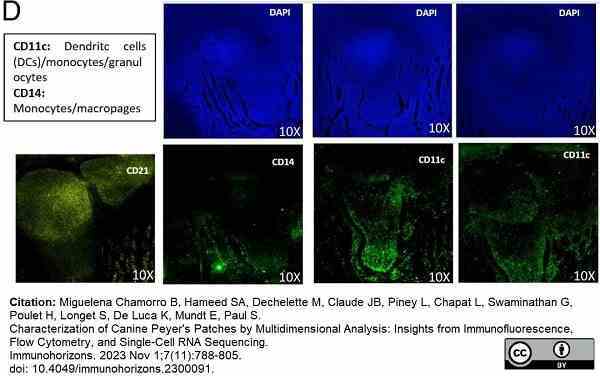
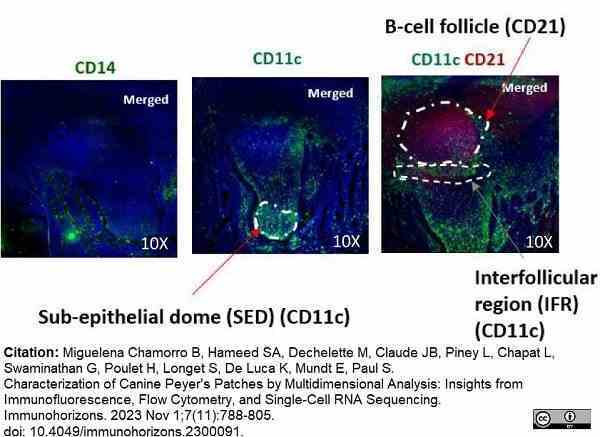
Miguelena Chamorro, B. et al. (2023) Characterization of Canine Peyer's Patches by Multidimensional Analysis: Insights from Immunofluorescence, Flow Cytometry, and Single-Cell RNA Sequencing.
Immunohorizons. 7 (11): 788-805. -
Mason, N.J. et al. (2021) Development of a fully canine anti-canine CTLA4 monoclonal antibody for comparative translational research in dogs with spontaneous tumors.
MAbs. 13 (1): 2004638.
Further Reading
-
Bazil, V. et al. (1986) Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen.
Eur J Immunol. 16:1583-9. -
Simmons, D. L. et al. (1989) Monocyte antigen CD14 is a phospholipid anchored membrane protein.
Blood. 73:284-9. -
Pugin, J. et al. (1994) CD14 is a pattern recognition receptor.
Immunity.1:509-16. -
Dziarski, R. et al. (1998) Binding of bacterial peptidoglycan to CD14.
J Biol Chem. 273:8680-90. -
Jersmann, H.P. (2005) Time to abandon dogma: CD14 is expressed by non-myeloid lineage cells.
Immunol Cell Biol. 83:462-7. -
Piriou-Guzylack, L. (2008) Membrane markers of the immune cells in swine: an update.
Vet Res. 39: 54.
- UniProt
- P08571
- Entrez Gene
- CD14
- GO Terms
- GO:0005886 plasma membrane
- GO:0001530 lipopolysaccharide binding
- GO:0001847 opsonin receptor activity
- GO:0006915 apoptosis
- GO:0006909 phagocytosis
- GO:0006954 inflammatory response
- GO:0008063 Toll signaling pathway
- GO:0031225 anchored to membrane
- GO:0016019 peptidoglycan receptor activity
- View More GO Terms
- GO:0032760 positive regulation of tumor necrosis factor production
- GO:0045087 innate immune response
- GO:0070891 lipoteichoic acid binding
- GO:0071222 cellular response to lipopolysaccharide
- GO:0071223 cellular response to lipoteichoic acid
MCA1568SBV760
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Human ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up