CD4 antibody | vpg34




Mouse anti Cat CD4:FITC
- Product Type
- Monoclonal Antibody
- Clone
- vpg34
- Isotype
- IgG1
- Specificity
- CD4
| Mouse anti Cat CD4 antibody, clone vpg34 recognizes the feline homolog of the human CD4 antigen. CD4 is not expressed on feline monocytes. |
- Target Species
- Cat
- Product Form
- Purified IgG conjugated to Fluorescein Isothiocyanate Isomer 1 (FITC) - liquid
- Preparation
- Antibody purified from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
1% bovine serum albumin - Immunogen
- Immunoaffinity purified feline CD4.
- Approx. Protein Concentrations
- IgG concentration 0.1mg/ml
- Fusion Partners
- Spleen cells from immunized BALB/c were fused with cells of the NS0 mouse myeloma cell line.
- Max Ex/Em
-
Fluorophore Excitation Max (nm) Emission Max (nm) FITC 490 525 - Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended. This product is photosensitive and should be protected from light.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | Neat | 1/10 |
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 lymphocytes in 100μl
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG1 Negative Control:FITC | MCA1209F | F | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG1 Negative Control:FITC | ||||||
| Mouse IgG1 Negative Control:FITC | MCA928F | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG1 Negative Control:FITC | ||||||
References for CD4 antibody
-
Calmann, J.J. et al. (1993) Morphologic Characterization of the Lymph Node Changes in Feline Immunodeficiency Virus Infection as an Animal Model of AIDS1
In: Racz P, Letvin NL, Gluckman JC (eds): Animal Models of HIV and Other Retroviral Infections. Basel, Karger, 1993, pp 115-136 -
Willett, B.J. et al. (1994) The generation of monoclonal antibodies recognising novel epitopes by immunisation with solid matrix antigen-antibody complexes reveals a polymorphic determinant on feline CD4.
J Immunol Methods. 176 (2): 213-20. -
Hosie, M.J. et al. (2000) Vaccination with inactivated virus but not viral DNA reduces virus load following challenge with a heterologous and virulent isolate of feline immunodeficiency virus.
J Virol. 74: 9403-11. -
Hosie, M.J. et al. (2002) Evolution of replication efficiency following infection with a molecularly cloned feline immunodeficiency virus of low virulence.
J Virol. 76: 6062-72. -
Flynn, J.N. et al. (2002) Longitudinal analysis of feline leukemia virus-specific cytotoxic T lymphocytes: correlation with recovery from infection.
J Virol. 76: 2306-15. -
Campbell, D.J. et al. (2004) Insulin-like growth factor-I (IGF-I) and its association with lymphocyte homeostasis in the ageing cat.
Mech Ageing Dev. 125: 497-505. -
Campbell, D.J. et al. (2004) Age-related differences in parameters of feline immune status.
Vet Immunol Immunopathol. 100: 73-80. -
Milner, R.J. et al. (2004) Suppurative rhinitis associated with Haemophilus species infection in a cat.
J S Afr Vet Assoc. 75: 103-7.
View The Latest Product References
-
Webb, C. et al. (2006) Use of flow cytometry and monochlorobimane to quantitate intracellular glutathione concentrations in feline leukocytes.
Vet Immunol Immunopathol. 112 (3-4): 129-40. -
Veir, J.K. et al. (2006) Evaluation of a novel immunotherapy for treatment of chronic rhinitis in cats.
J Feline Med Surg. 8 (6): 400-11. -
Willett, B.J. et al. (2007) Probing the interaction between feline immunodeficiency virus and CD134 by using the novel monoclonal antibody 7D6 and the CD134 (Ox40) ligand.
J Virol. 81: 9665-79. -
Avery, P.R. et al. (2007) Sustained generation of tissue dendritic cells from cats using organ stromal cell cultures.
Vet Immunol Immunopathol. 117 (3-4): 222-35. -
Veir, J.K. et al. (2007) Effect of supplementation with Enterococcus faecium (SF68) on immune functions in cats.
Vet Ther. 8: 229-38. -
Webb, C. et al. (2008) Oxidative stress during acute FIV infection in cats.
Vet Immunol Immunopathol. 122 (1-2): 16-24. -
Carreño, A.D. et al. (2008) Loss of naïve (CD45RA+) CD4+ lymphocytes during pediatric infection with feline immunodeficiency virus.
Vet Immunol Immunopathol. 121: 161-8. -
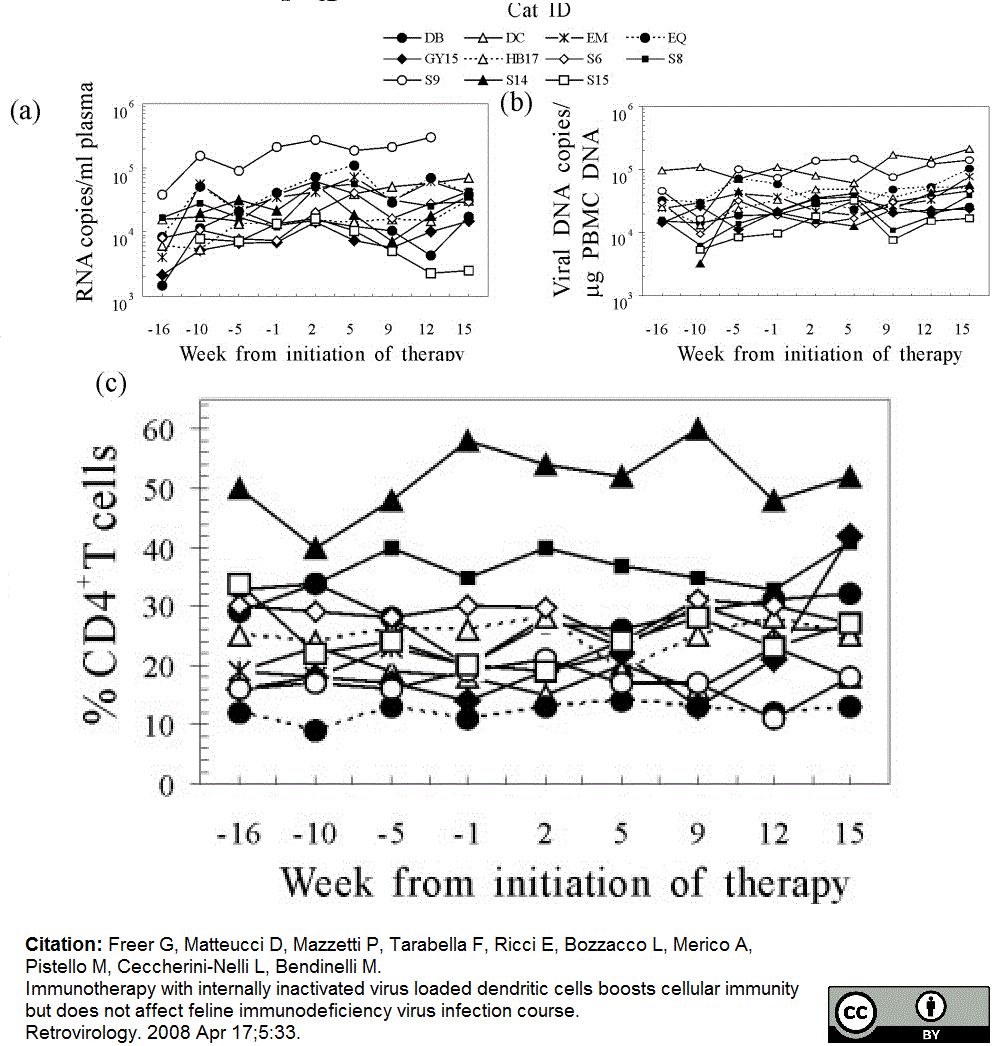
Freer, G. et al. (2008) Immunotherapy with internally inactivated virus loaded dendritic cells boosts cellular immunity but does not affect feline immunodeficiency virus infection course.
Retrovirology. 5: 33. -
Reinero, C.R. et al. (2008) Adjuvanted rush immunotherapy using CpG oligodeoxynucleotides in experimental feline allergic asthma.
Vet Immunol Immunopathol. 121: 241-50. -
Reche, A.Jr. et al. (2010) Cutaneous mycoflora and CD4:CD8 ratio of cats infected with feline immunodeficiency virus.
J Feline Med Surg. 12: 355-8. -
Kraase, M. et al. (2010) Feline immunodeficiency virus env gene evolution in experimentally infected cats.
Vet Immunol Immunopathol. 134: 96-106. -
Pistello, M. et al. (2010) Env-expressing autologous T lymphocytes induce neutralizing antibody and afford marked protection against feline immunodeficiency virus.
J Virol. 84: 3845-56. -
Novacco, M. et al. (2012) Protection from reinfection in "Candidatus Mycoplasma turicensis"-infected cats and characterization of the immune response.
Vet Res. 43: 82. -
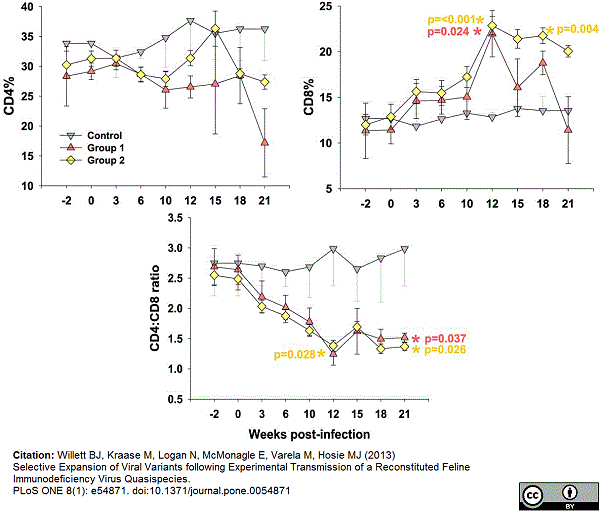
Willett, B.J. et al. (2013) Selective expansion of viral variants following experimental transmission of a reconstituted feline immunodeficiency virus quasispecies.
PLoS One. 8: e54871. -
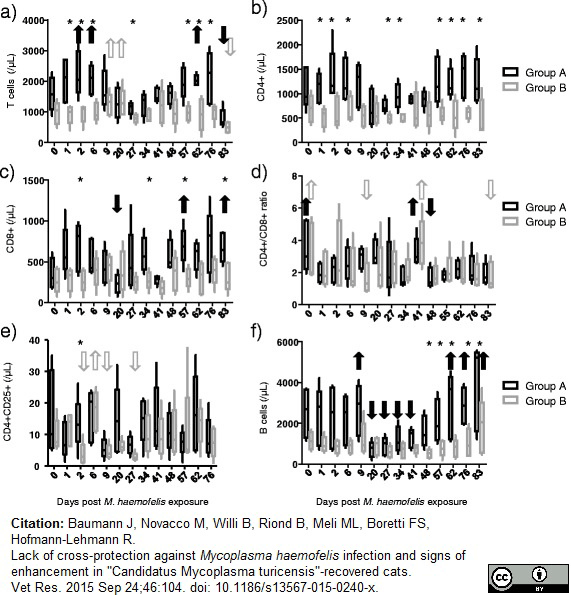
Baumann J et al. (2015) Lack of cross-protection against Mycoplasma haemofelis infection and signs of enhancement in "Candidatus Mycoplasma turicensis"-recovered cats.
Vet Res. 46: 104. -
McLuckie, A.J. et al. (2016) Detection of Felis catus gammaherpesvirus 1 (FcaGHV1) in peripheral blood B- and T-lymphocytes in asymptomatic, naturally-infected domestic cats.
Virology. 497: 211-6. -
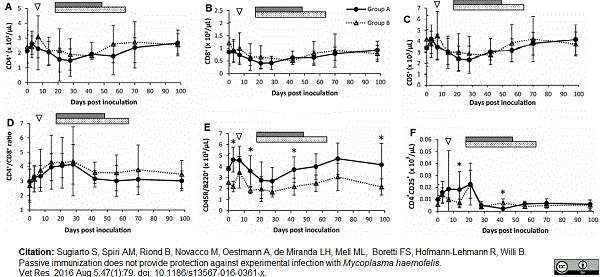
Sugiarto, S. et al. (2016) Passive immunization does not provide protection against experimental infection with Mycoplasma haemofelis.
Vet Res. 47 (1): 79. -
MirandaL, H.M. et al. (2018) Co-infection with feline retrovirus is related to changes in immunological parameters of cats with sporotrichosis.
PLoS One. 13 (11): e0207644. -
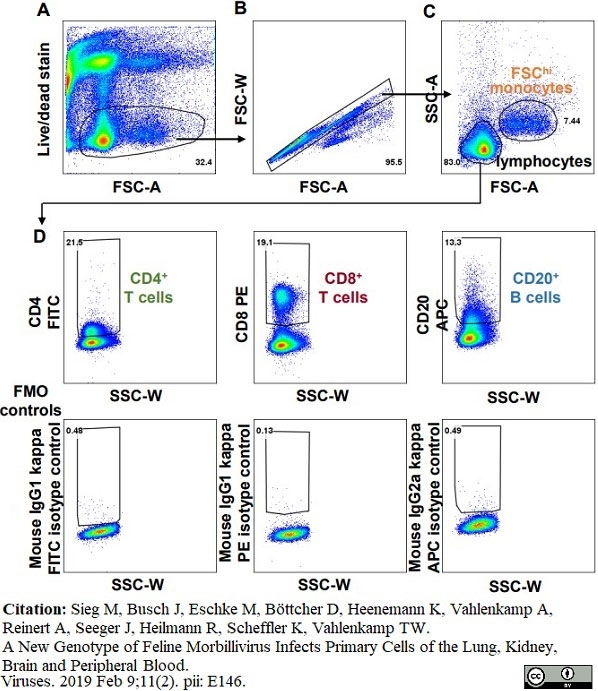
Sieg, M. et al. (2019) A New Genotype of Feline Morbillivirus Infects Primary Cells of the Lung, Kidney, Brain and Peripheral Blood.
Viruses. 11 (2) Feb 09 [Epub ahead of print]. -
Maeta, N. et al. (2019) Lymphokine-activated killer cell transplantation after anti-cancer treatment in two aged cats.
Open Vet J. 9 (2): 147-50. -
de Oliveira Matheus, L.F. et al. (2021) Effects of Saccharomyces cerevisiae. cell wall addition on feed digestibility, fecal fermentation and microbiota and immunological parameters in adult cats.
BMC Vet Res. 17 (1): 351. -
Rütgen, B.C. et al. (2022) Composition of lymphocyte subpopulations in normal and mildly reactive peripheral lymph nodes in cats.
J Feline Med Surg. 24 (2): 77-90. -
Cha, S. et al. (2023) Non-B, Non-T Acute Lymphoblastic Leukemia in a Cat
Journal of Veterinary Clinics. 40 (4): 298-302.
- RRID
- AB_322909
MCA1346F
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Cat ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up