-
US | en

Optics and Detection
After hydrodynamic focusing, each particle passes through one or more beams of focused light. Light scattering or fluorescence emission (from autofluorescence or if the particle is labeled with a fluorophore) provides information about the particle’s properties. Lasers are the most commonly used light sources in flow cytometry.
Lasers produce a single wavelength of light (a laser line) at a specific frequency. They are available at different wavelengths ranging from ultraviolet to far red and have a variable range of power levels (photon output/time typically specified in mW).
Forward Scatter
Light that is scattered in the forward direction after interacting with a particle, typically up to 20o offset from the laser beam's axis, is collected by a photomultiplier tube (PMT) or photodiode and is known as the forward scatter (FSC) channel. This angle can however vary depending on your instrument, leading to variation of FSC signals between different machines. This FSC measurement can give an estimation of a particle's size with larger particles refracting more light than smaller particles, but this can depend on several factors such as the sample, the wavelength of the laser, the collection angle and the refractive index of the sample and sheath fluid. A good example of this is in the detection of small particles. When the particles are smaller than the wavelength of the illumination source, e.g. a 200 nm exosome using a 488 nm laser the light is not necessarily scattered in a forward direction.
Side Scatter
Light measured at a 90o angle to the excitation line is called side scatter (SSC). The SSC can provide information about the relative complexity (for example, granularity and internal structures) of a cell or particle; however as with forward scatter this can depend on various factors. Both FSC and SSC are unique for every particle and a combination of the two may be used to roughly differentiate cell types in a heterogeneous population such as blood. However, this depends on the sample type and the quality of sample preparation, so fluorescent labeling is generally required to obtain more detailed information.
Detectors and Filters
Fluorescence measurements taken at different wavelengths can provide quantitative and qualitative data about fluorophore-labeled cell surface receptors or intracellular molecules such as DNA and cytokines. Most flow cytometers use separate channels and detectors to detect emitted light, the number of which vary according to the instrument and the manufacturer. Detectors are either photomultiplier tubes or avalanche photodiodes (APD). PMTs are the most commonly used detectors.
The specificity of detection is controlled by optical filters, which block certain wavelengths whilst transmitting (passing) others. There are three major filter types. Long pass filters allow light through above a cutoff wavelength; short pass filters permit light below a certain wavelength and band pass filters transmit light within a specified narrow range of wavelengths (termed a band width). These dichroic filters can block light by phased reflection allowing certain light to pass through and interfering with other wavelengths (Figure 2).
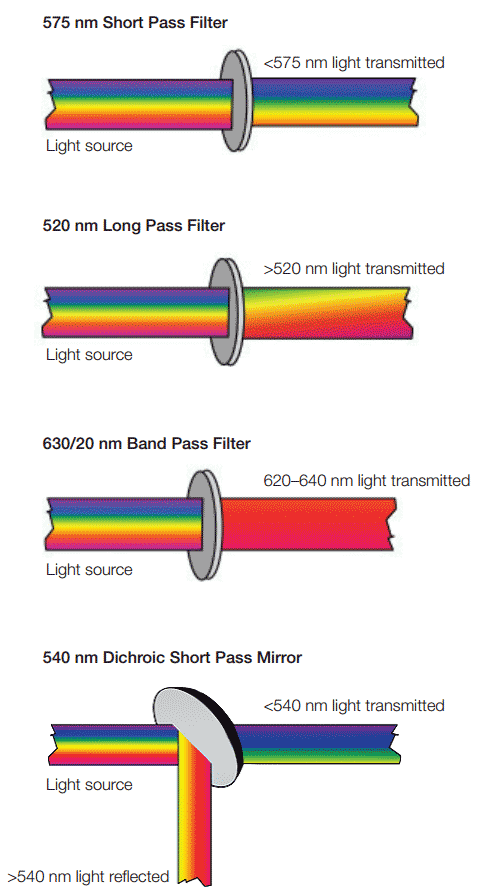
Fig. 2. Different types of optical filters
A dichroic filter is also a mirror when placed at an angle to the oncoming light. This type of filter can now perform two functions. First, it allows specific wavelengths to pass in the forward direction, second it can reflect light at a 90o angle. This allows the light path to be passed through a series of filters. The precise choice and order of the filters can be arranged so that multiple signals can be detected simultaneously (Figure 3).
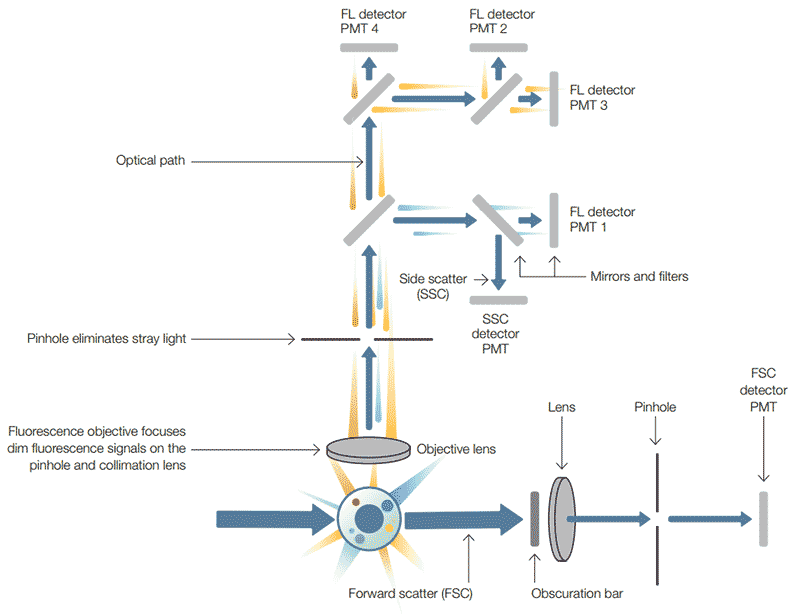
Fig. 3. Schematic overview of a typical flow cytometer setup. FL, fluorescence; PMT, photomultiplier tube; SSC, side scatter; FSC, forward scatter; blue arrow, light path.
