-
US | en

Antibody Titration
Another important consideration when building multicolor panels is titration of your antibodies. Excess antibody will bind at low affinity and create background that will reduce the resolution and therefore cloud your results. In addition too much antibody may result in a false negative prozone effect. It is therefore important to determine the right amount of antibody needed for your specific sample. If you use isotype controls, be sure to use them at the same concentration. To determine the best antibody concentration, the stain index, which is defined as the ratio of the separation between the positive and negative population divided by two times the standard deviation of the negative population, can be used as a guide.
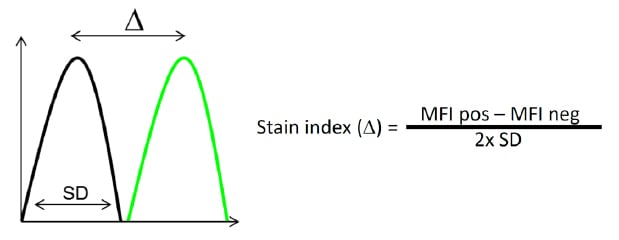
Fig. 30. Stain index. The stain index is the ratio of the separation between the positive population (green) and the negative population (black), divided by two times the standard deviation of the negative population.
Titration requires dilutions of antibody to be made and the same number of cells stained in the same volume. The dilution that represents the best stain index is the dilution to use. In the graph below, the points in the green box (Figure 31) represent the best concentrations that will generate specific staining with the least amount of background. Titration of antibodies can therefore improve your experiments and could save you money by using a smaller antibody concentration than the one stated in the manufacturer's instructions.
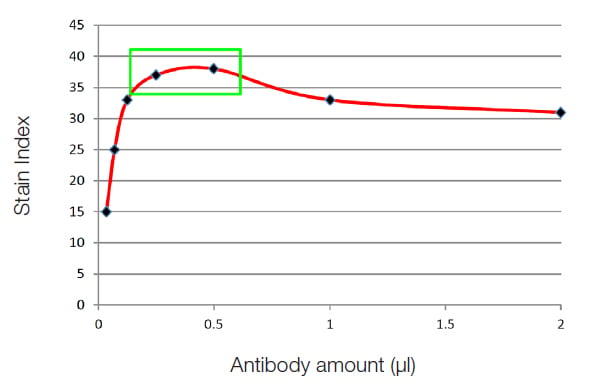
Fig. 31. Antibody titration. Plotting the stain index for each concentration of antibody will allow you to titrate the optimal amount of antibody for your experiment.
For more in-depth information on Antibody Titration see our Antibody Titration in Flow Cytometry page.
Useful Tools
There are useful tools that can help with panel design. Spectra viewers will help you determine the amount of spillover and excitation by each laser. Relative brightness tables will give you information on pairing targets with fluorophores and marker expression data will help to determine expression patterns. Panel building websites can help with panel design and there are published examples of optimized multicolor immunofluorescence panels (OMIPS) which will also help with your panel design.
Finally, remember compromises will have to be made due to fluorophore availability, the cytometer you have, the cells you are working with and the antibodies available. Several combinations may be required but following these rules should reduce the time and effort required.
