Collagen I

Native Collagen I (Tail Tendon)
- Product Type
- Purified Protein
- Specificity
- Collagen I
- Region
- (TAIL TENDON)
| Native Murine collagen I is purified Mouse collagen I from tail tendon. Thermal denaturation converts the collagen to gelatin. Impurities: Mouse collagen type III 10% Mouse collagen (other types) <1% Non-collagenous proteins <0.5% |
- Target Species
- Mouse
- Product Form
- Purified Protein - liquid
- Preparation
- Collagens were extracted from washed dissected tissue into dilute acetic acid after mild pepsin treatment. Collagen type I was purified by using differential salt precipitation.
- Buffer Solution
- 0.5M acetic acid
- Preservative Stabilisers
- None present
- Purity
- 90%< by SDS PAGE (cross linked collagen type I dimers and trimers represent ~10%)
- Approx. Protein Concentrations
- 1.0 mg/ml
- Protein Molecular Weight
- ~300 kDa
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Storage in frost-free freezers is not recommended.
This product should be stored undiluted. Avoid repeated freezing and thawing as this may denature the protein. Should this product contain a precipitate we recommend microcentrifugation before use.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA |
References for Collagen I
-
Sebinger, D.D. et al. (2013) ECM modulated early kidney development in embryonic organ culture.
Biomaterials. 34 (28): 6670-82. -
Takahashi, S. et al. (2015) C-type lectin-like domain and fibronectin-like type II domain of phospholipase A2 receptor 1 modulate binding and migratory responses to collagen.
FEBS Lett. 589 (7): 829-35. -
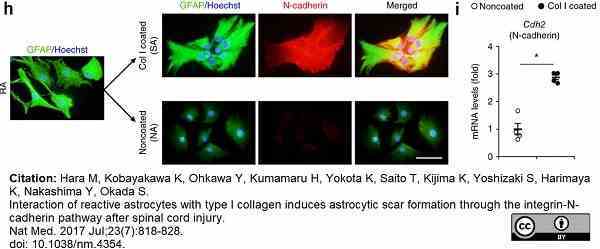
Hara, M. et al. (2017) Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury.
Nat Med. 23 (7): 818-28. -
Tamaru, T. et al. (2023) Glial scar survives until the chronic phase by recruiting scar-forming astrocytes after spinal cord injury.
Exp Neurol. 359: 114264.
Further Reading
-
Rhodes, R.K. & Miller, E.J. (1978) Physicochemical characterization and molecular organization of the collagen A and B chains.
Biochemistry. 17 (17): 3442-8.
- UniProt
- P11087
- Q01149
- Entrez Gene
- Col1a1
- Col1a2
- GO Terms
- GO:0001568 blood vessel development
- GO:0001649 osteoblast differentiation
- GO:0001957 intramembranous ossification
- GO:0001958 endochondral ossification
- GO:0005201 extracellular matrix structural constituent
- GO:0005584 collagen type I
- GO:0005737 cytoplasm
- GO:0010812 negative regulation of cell-substrate adhesion
- GO:0015031 protein transport
- View More GO Terms
- GO:0060325 face morphogenesis
- GO:0060346 bone trabecula formation
- GO:0060351 cartilage development involved in endochondral bone morphogenesis
- GO:0070208 protein heterotrimerization
- GO:0046332 SMAD binding
2150-1425
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Mouse ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up