Mouse Isotyping Kit

Mouse Monoclonal Antibody Isotyping Test Kit
- Product Type
- Kits
- Specificity
- Mouse Isotyping Kit
| The mouse monoclonal antibody isotyping test kit shows no cross-reactivity with bovine IgG (<0.1 %). |
- Target Species
- Mouse
- Reagents In The Kit
- 1 Desiccant vial containing mouse isotyping test strips, 10 tests.
10 Capped ready-to-use lyophilized microparticle development tubes. - Test Principle
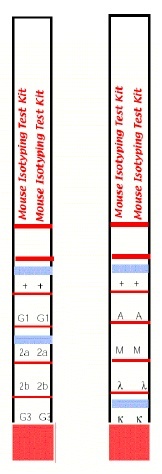
- The assay principle is based on anti-mouse kappa and anti-mouse lambda antibodies coupled onto coloured micro particles and equally reactive to any mouse monoclonal antibody regardless of its isotype. The isotyping strip has immobilized bands of goat anti-mouse antibodies corresponding to each of the common mouse antibody isotypes (IgG1, IgG2a, IgG2b, IgG3, IgM, and IgA) and to the kappa and lambda light chains. Both sides of the strip bear a positive flow control band, which indicates that the antibody-coated coloured micro particles have migrated through the strip. By using these two components, a mouse monoclonal antibody can be screened for isotype by simply diluting the antibody sample, pipetting the diluted sample into the development tube where it forms a complex with the antibody coated micro particles, and inserting the strip. This complex flows through the strip until it is bound by the immobilized goat anti-mouse antibody specific for the monoclonal’s isotype and its light chain. In approximately 5-10 minutes, the micro particle complex will aggregate as blue bands in the two sections corresponding to the monoclonal antibody's isotype and its light chain. Development of the strip is complete when the positive flow control band on each side of the test strip turns blue. Please note, this kit is not suitable for use with antibodies of the isotype IgG2c; some cross-reactivity may be observed with isotype IgG2a.
- Regulatory
- For research purposes only
- Guarantee
- Guaranteed until date of expiry. Please see product label.
Do not use components from different lots.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Isotyping Assay |
- Technical Advice
- PROBLEM
No heavy and or light-chain band appeared on the strip, but the positive flow control bands appeared.
Possible causes:
1. The antibody concentration was too low - prepare a less dilute sample and re-test.
2. No antibody was in the sample - the hybridoma is either not secreting or is not a mouse monoclonal. If possible sub-clone the hybridoma and re-test.
3. Freshly diluted samples were not used - prepare fresh dilutions and re-test.
PROBLEM
Multiple heavy and light-chain bands appear on the strip.
Possible causes:
1. Antibody concentration was too high - dilute sample further and re-test.
2. For ascites, there may be small amounts of contaminating antibodies produced - dilute sample further and re-test.
3. For tissue culture supernatant, a mixed culture may be present - re-clone the hybridoma and re-test.
PROBLEM
No positive flow control bands appear.
Possible causes:
1. Sample volume was too low (<150ul) - carefully dilute a fresh sample and pipette 150ul into a new development tube and re-test.
2. Strip removed from development tube too early - re-test and allow strip to react for at least 10 minutes. - Instructions For Use
- Note: All reagents should be brought to room temperature before use.
Sample Preparation:
Dilute all monoclonal antibody samples to a concentration of 1.0 ug/ml in PBS containing 1% w/v bovine serum albumin (BSA). If the concentration of the sample is entirely unknown, make dilutions based on the following estimates:
Typically, serum contains between 10-15 mg/ml IgG and ascites can be as high as 10 mg/ml. Hollow fibre bioreactor culture supernatants contain approximately 0.5-1.0 mg/ml, whereas static flask tissue culture supernatants usually contain 10-50 ug/ml. Using these estimates, the appropriate dilutions can be made.
150ul of the diluted sample will be added to the development tubes.
Assay Protocol:
1. Remove the required number of isotyping strips from the desiccant vial and replace the cap. Remove the caps from an equal number of development tubes.
Note: the tubes may be labeled with a marker for identification.
2. Pipette 150ul of the freshly diluted sample into each development tube and incubate at room temperature for 30 seconds. Vortex the tube briefly to ensure that the coloured micro particle solution is completely re-suspended.
3. Place one isotyping strip, with the solid red end at the bottom, into each development tube.
Interpretation of Results:
Interpret the results at 5-10 minutes once the positive flow control bands have appeared. Within 5-10 minutes, a blue band will appear above the letters in one of the class or subclass windows as well as in either the kappa or lambda window of the strip, indicating the heavy and light-chain composition of the monoclonal antibody. The intensity of the blue bands will increase as the sample continues to flow up the strip. The positive flow control bands on each side of the isotyping test strip should also appear, indicating that the antibody-coated micro particles are functional and have flowed up the strip. In cases where the sample is very dilute, the development time may take up to 10 minutes.
Note: For a permanent experimental record or for an easier interpretation of results when testing multiple samples, the solid red area may be cut off the bottom of the strip to prevent further band development once the positive flow control bands have appeared. A gentle stream of air can be applied to the membrane portion of the strip to assist in drying the membrane and preventing any further development. Do not wash the strip to stop the reaction.
References for Mouse Isotyping Kit
-
Kimura, H. et al. (2008) The organization of histone H3 modifications as revealed by a panel of specific monoclonal antibodies.
Cell Struct Funct. 33: 61-73. -
Gilbert, J.A. et al. (2006) Monoclonal pathogenic antibodies to the thyroid-stimulating hormone receptor in Graves' disease with potent thyroid-stimulating activity but differential blocking activity activate multiple signaling pathways.
J Immunol. 176 (8): 5084-92. -
Keim, S.A. et al. (2008) Generation and characterization of monoclonal antibodies against the proregion of human desmoglein-2.
Hybridoma (Larchmt). 2008 Aug;27(4): 249-58. -
Stenman, U.H. et al. (2011) Elimination of complement interference in immunoassay of hyperglycosylated human chorionic gonadotropin.
Clin Chem. 57: 1075-7. -
Hayashi-Takanaka, Y. et al. (2011) Tracking epigenetic histone modifications in single cells using Fab-based live endogenous modification labeling.
Nucleic Acids Res. 39: 6475-6488. -
Nakayama, E. et al. (2011) Antibody-dependent enhancement of Marburg virus infection.
J Infect Dis. 204 Suppl 3: S978-85. -
Urán, M.E. et al. (2011) Detection of antibodies against Paracoccidioides brasiliensis melanin in in vitro and in vivo studies during infection.
Clin Vaccine Immunol. 18: 1680-8. -
Hayashi-Takanaka, Y. et al. (2015) Distribution of histone H4 modifications as revealed by a panel of specific monoclonal antibodies.
Chromosome Res. 23 (4): 753-66.
View The Latest Product References
-
Álvarez, B. et al. (2015) Molecular and functional characterization of porcine Siglec-3/CD33 and analysis of its expression in blood and tissues.
Dev Comp Immunol. 51 (2): 238-50. -
Leng J et al. (2014) Development of a novel anti ESAT-6 monoclonal antibody for screening of Mycobacterium tuberculosis.
Int J Clin Exp Med. 7 (11): 4238-43. -
Broecker, F. et al. (2016) Multivalent display of minimal Clostridium difficile glycan epitopes mimics antigenic properties of larger glycans.
Nat Commun. 7: 11224. -
Arimitsu, H. et al. (2017) Immunochromatographic detection of the heat-labile enterotoxin of enterotoxigenic Escherichia coli with cross-detection of cholera toxin
Journal of Microbiological Methods. 132: 148-52. -
Okda, F. et al. (2015) Development of an indirect ELISA, blocking ELISA, fluorescent microsphere immunoassay and fluorescent focus neutralization assay for serologic evaluation of exposure to North American strains of Porcine Epidemic Diarrhea Virus.
BMC Vet Res. 11: 180. -
Poderoso, T. et al. (2019) Analysis of the expression of porcine CD200R1 and CD200R1L by using newly developed monoclonal antibodies.
Dev Comp Immunol. 100: 103417. -
Maeda, H. et al (2022) Urinary kidney injury molecule-1 as early diagnostic marker of chronic kidney disease in cats
Medical Mass Spec 6 (1): 1-9. -
Chen, J.Y. et al. (2020) Properdin Is a Key Player in Lysis of Red Blood Cells and Complement Activation on Endothelial Cells in Hemolytic Anemias Caused by Complement Dysregulation.
Front Immunol. 11: 1460.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Mouse ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up