Western Blot: Indirect vs. Direct Detection
Overview
Antibody detection of the target protein is accomplished using a one-step or two-step protocol. The one-step procedure, direct detection, relies upon a single antibody which has been covalently joined to an easily detected label molecule (biotin, an enzyme, or a fluorescent dye). Labeled primary antibodies can be ordered directly from Bio-Rad or other commercial antibody suppliers. In addition, it is possible to directly label an antibody by using a commercially supplied labeling kit such as our LYNX Rapid Conjugation Kits®, or with in-house reagents.
With indirect detection, two different antibodies are used in sequence for the detection step. First, the Western blot is incubated with an unlabeled primary antibody directed against the target protein. After washing, a labeled secondary antibody is used to detect the presence of the primary antibody, and thus the target protein. The labeled secondary antibody is typically directed against the immunoglobulin class or subclass of the primary antibody’s species. For example, one of our popular secondaries is STAr88P, a purified donkey antibody raised against goat/sheep immunoglobulin (IgG) which is coupled to an HrP label. Biotinylated primary antibodies also require a two-step detection procedure; however, the second step involves incubation with streptavidin, a bacterial protein, conjugated to HrP (or AP), rather than with a labeled antibody.
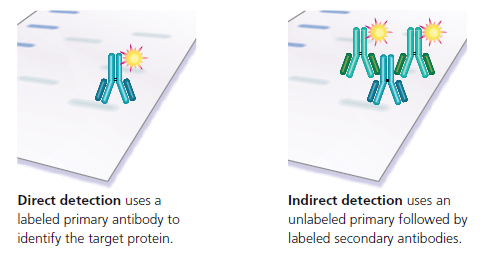
Figure 11: Direct vs. Indirect Detection.
Comparison of Direct and Indirect Detection Methods
| Direct Detection | Indirect Detection |
|---|---|
| Advantages | Advantages |
|
|
| Disadvantages | Disadvantages |
|
|
| Immunodetection: Blocking and Antibody Incubation | Detection with Substrate |





