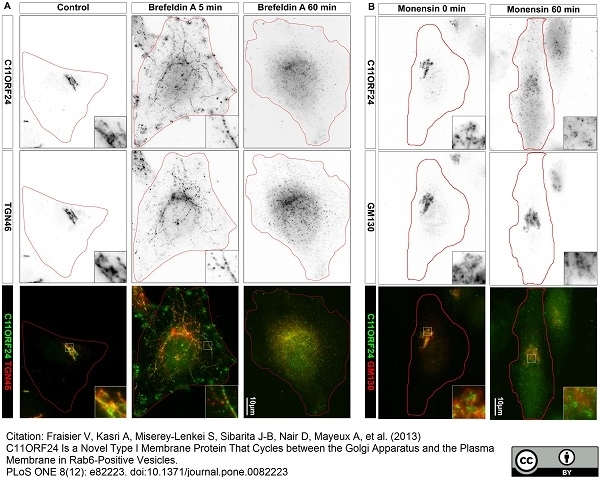
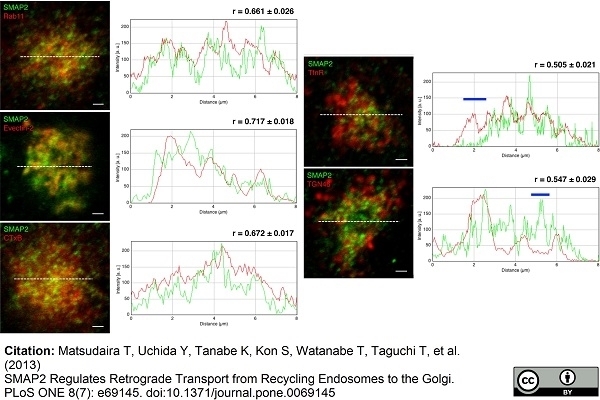
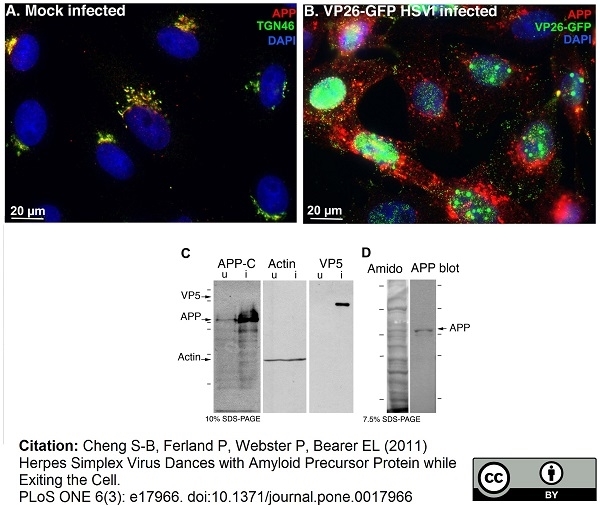
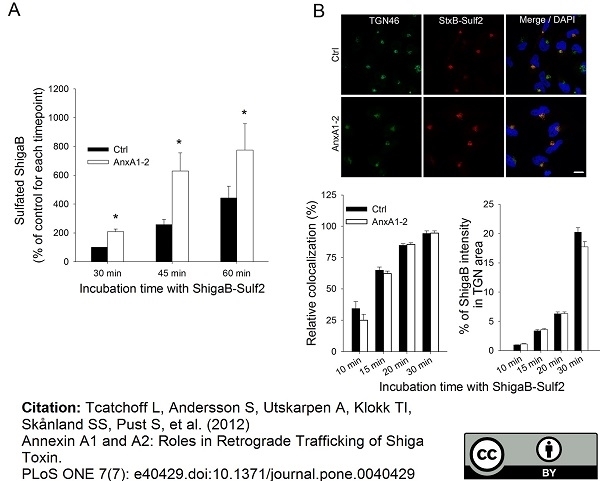
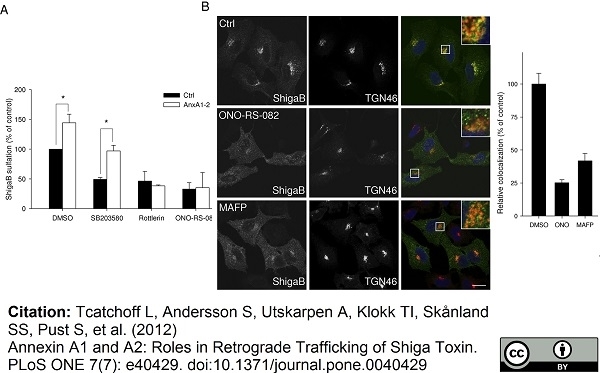
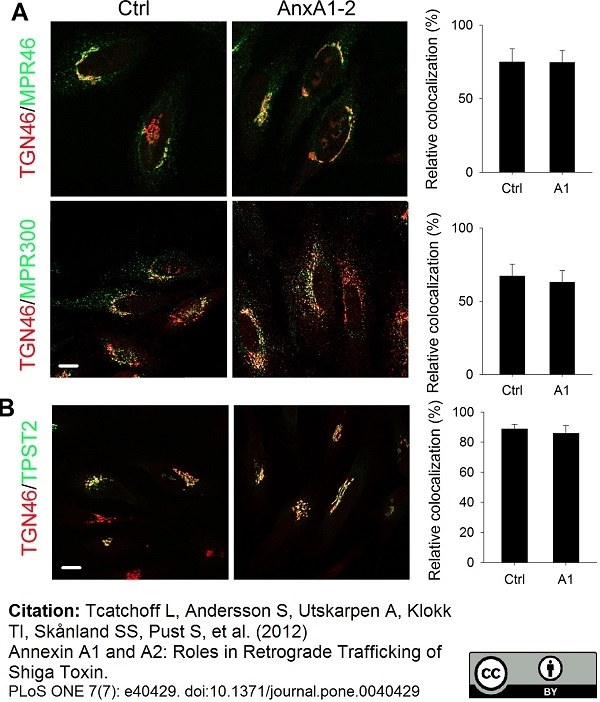
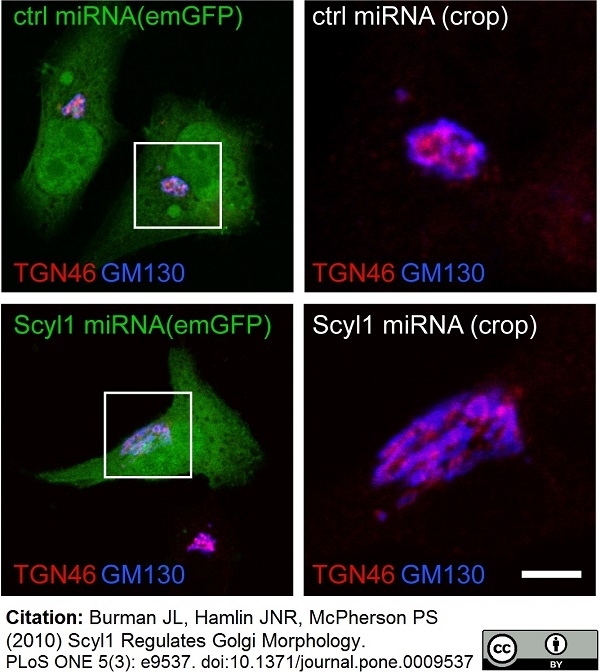
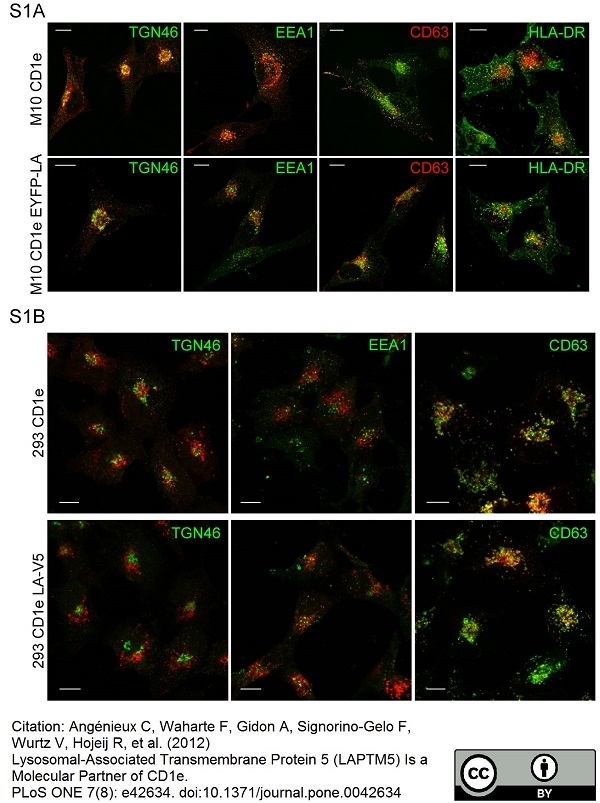
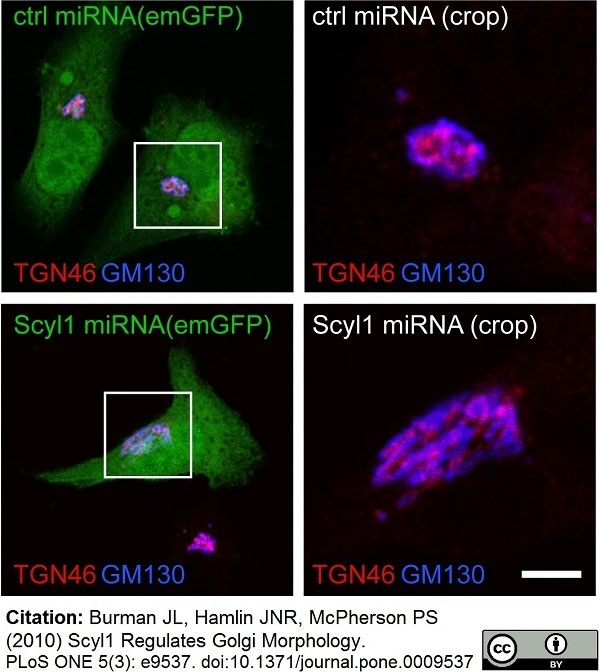
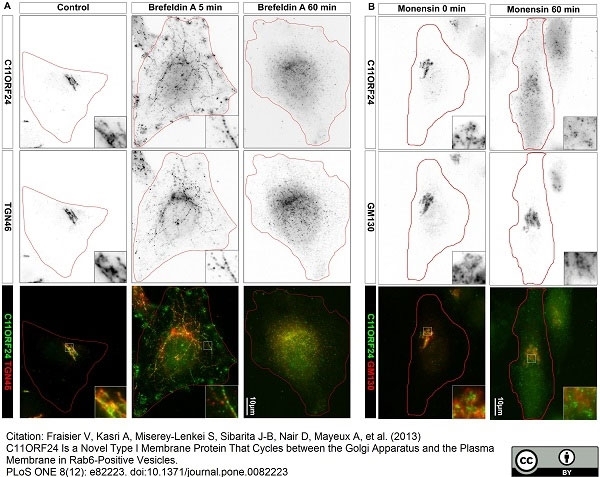
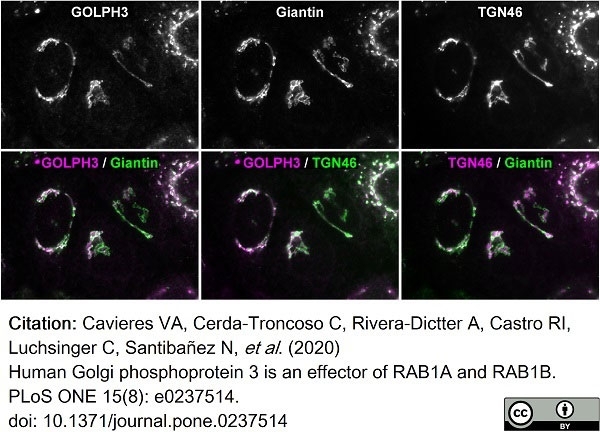
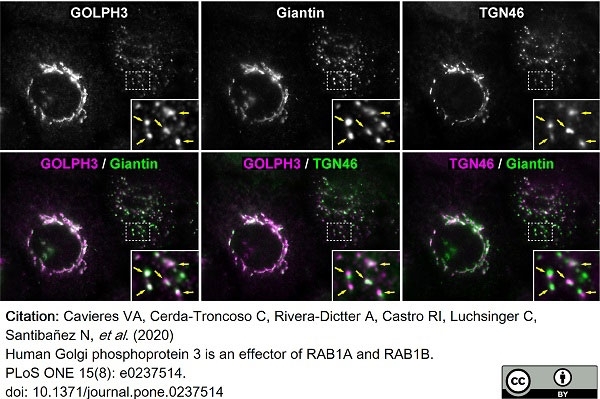
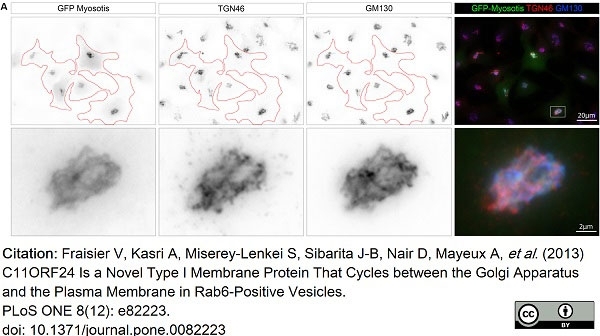
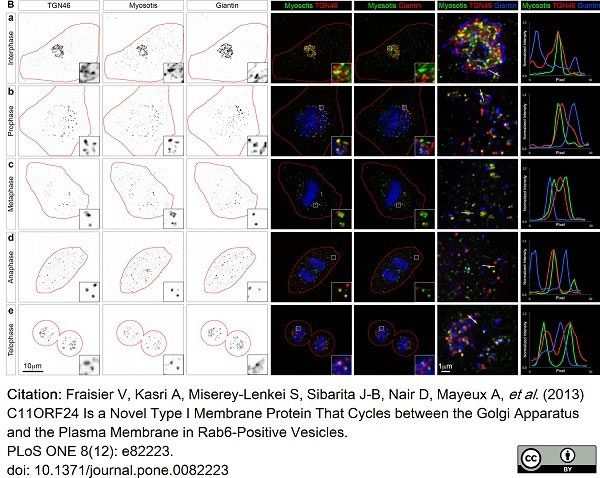
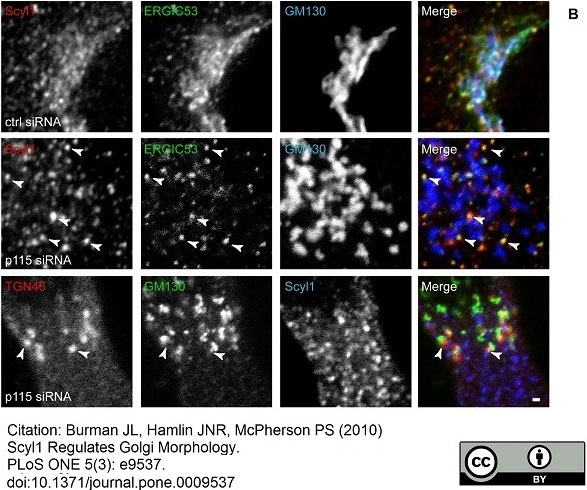
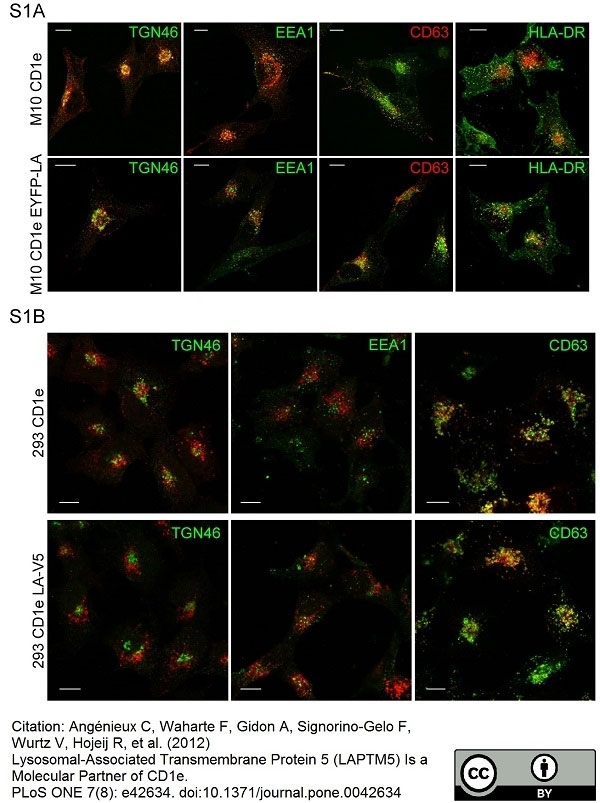
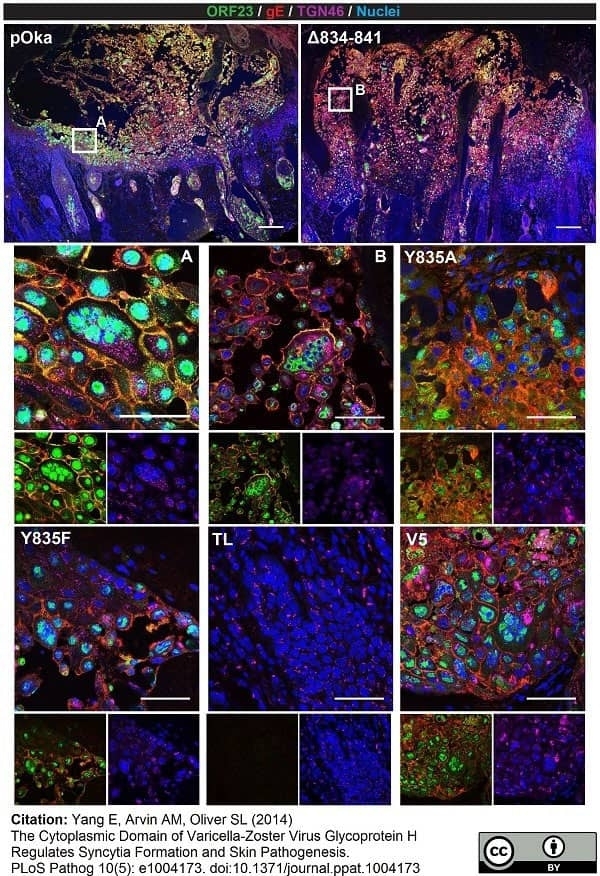
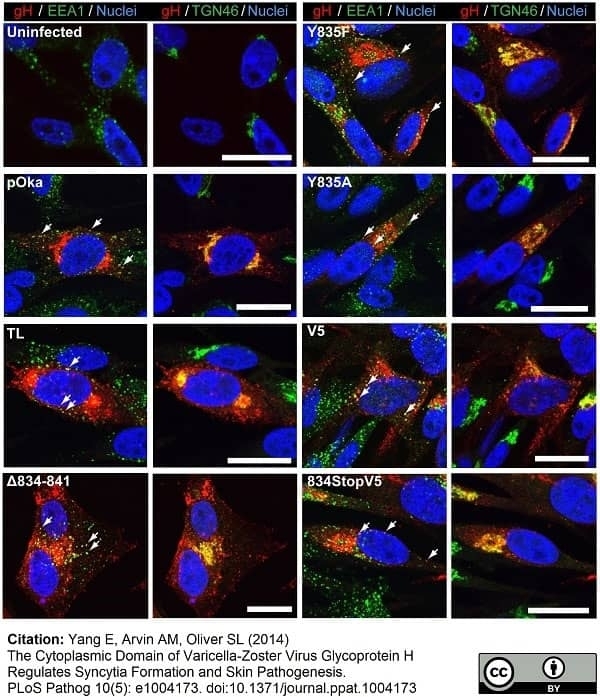
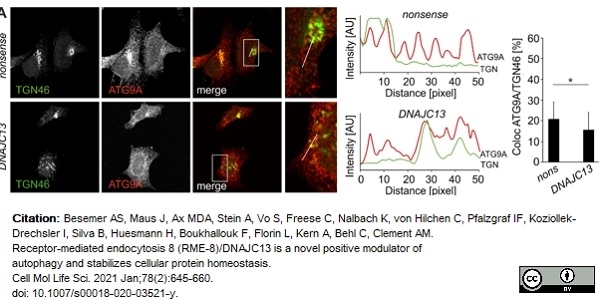
TGN46 antibody



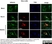


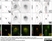

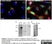
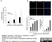

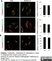
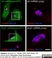
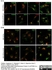

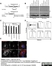





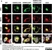


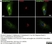
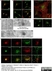
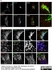
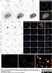

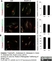
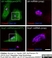
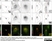


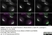


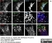
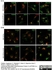

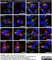



















Sheep anti Human TGN46
- Product Type
- Polyclonal Antibody
- Isotype
- Polyclonal IgG
- Specificity
- TGN46
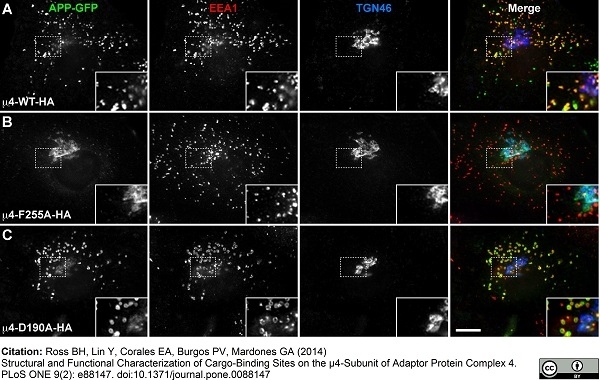
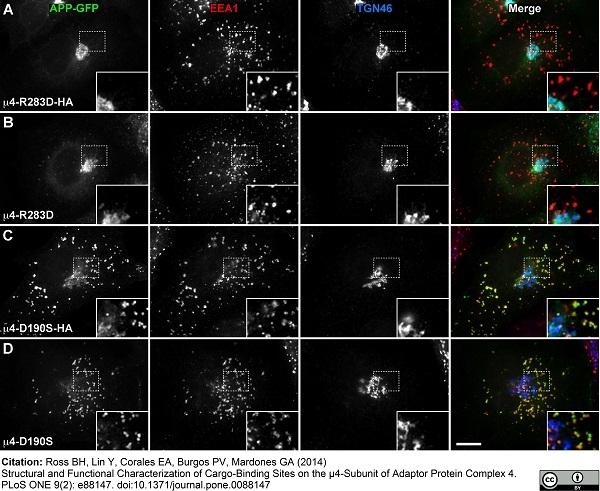
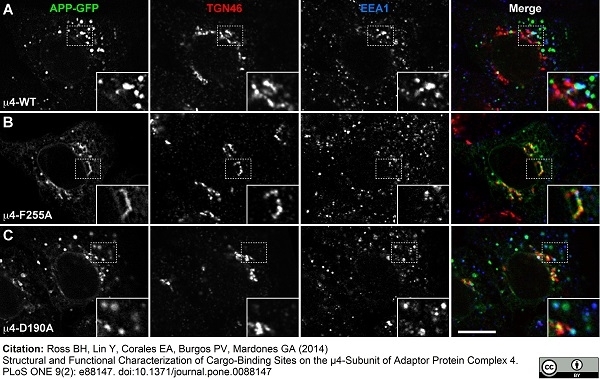
| Sheep anti Human TGN46 antibody recognizes trans-Golgi network integral membrane protein 2 (TGOLN2), also known as TGN38 homolog, TGN46, TGN48 or trans-Golgi network protein TGN51. TGN46 is a 437 amino acid glycoprotein localized to the trans-Golgi network. TGN46 has been reported as being the best available marker for human trans-Golgi network. TGN46 is a heavily glycosylated protein of around 110-120 kDa. Multiple isoforms of TGN46 are generated by alternative splicing differing in sequence at the C-terminal portion. Sheep anti Human TGN46 antibody is expected to recognize all identified isoforms. |
- Target Species
- Human
- Species Cross-Reactivity
-
Target Species Cross Reactivity Primate - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG - liquid
- Antiserum Preparation
- Antisera to human TGN46 were raised by repeated immunisation of sheep with highly purified antigen. Purified IgG prepared by affinity chromatography.
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- <0.1% Sodium Azide (NaN3)
0.5% Bovine Serum Albumin
25% Glycerol - Immunogen
- Recombinant human TGN46.
- Approx. Protein Concentrations
- IgG concentration 0.25 mg/ml
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Immunofluorescence | |||
| Immunohistology - Frozen 1 | 0.1ug/ml | 1ug/ml | |
| Western Blotting | 0.1ug/ml | 1ug/ml |
- 1Fixation with 3% paraformaldehyde or methanol/acetone is recommended.
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Rabbit anti Sheep IgG (H/L):Biotin | 5184-2304 | C E F WB | 1.5 ml |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rabbit anti Sheep IgG (H/L):Biotin | ||||||
References for TGN46 antibody
-
Prescott AR et al. (1997) Distinct compartmentalization of TGN46 and beta 1,4-galactosyltransferase in HeLa cells.
Eur J Cell Biol. 72 (3): 238-46. -
van Dam, E.M. et al. (2002) Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles.
Mol Biol Cell. 13: 169-82. -
Salahpour, A. et al. (2004) Homodimerization of the beta2-adrenergic receptor as a prerequisite for cell surface targeting.
J Biol Chem. 279 (32): 33390-7. -
Drakesmith, H. et al. (2005) HIV-1 Nef down-regulates the hemochromatosis protein HFE, manipulating cellular iron homeostasis.
Proc Natl Acad Sci U S A. 102 (31): 11017-22. -
Mills, I.G. et al. (2005) Huntingtin interacting protein 1 modulates the transcriptional activity of nuclear hormone receptors.
J Cell Biol. 170 (2): 191-200. -
Mills, I.G. et al. (2005) Huntingtin interacting protein 1 modulates the transcriptional activity of nuclear hormone receptors.
J Cell Biol. 170: 191-200. -
Vuillier, F. et al. (2005) Lower levels of surface B-cell-receptor expression in chronic lymphocytic leukemia are associated with glycosylation and folding defects of the mu and CD79a chains.
Blood. 105 (7): 2933-40. -
Berarducci, B. et al. (2006) Essential functions of the unique N-terminal region of the varicella-zoster virus glycoprotein E ectodomain in viral replication and in the pathogenesis of skin infection.
J Virol. 80: 9481-96.
View The Latest Product References
-
Edwards, T.L. et al. (2009) Endogenous spartin (SPG20) is recruited to endosomes and lipid droplets and interacts with the ubiquitin E3 ligases AIP4 and AIP5.
Biochem J. 423 (1): 31-9. -
Esk, C. et al. (2010) The clathrin heavy chain isoform CHC22 functions in a novel endosomal sorting step.
J Cell Biol. 188: 131-44. -
Vleck, S.E. et al. (2010) Anti-glycoprotein H antibody impairs the pathogenicity of varicella-zoster virus in skin xenografts in the SCID mouse model.
J Virol. 84: 141-52. -
Sadaoka, T. et al. (2010) Characterization of the varicella-zoster virus ORF50 gene, which encodes glycoprotein M.
J Virol. 84: 3488-502. -
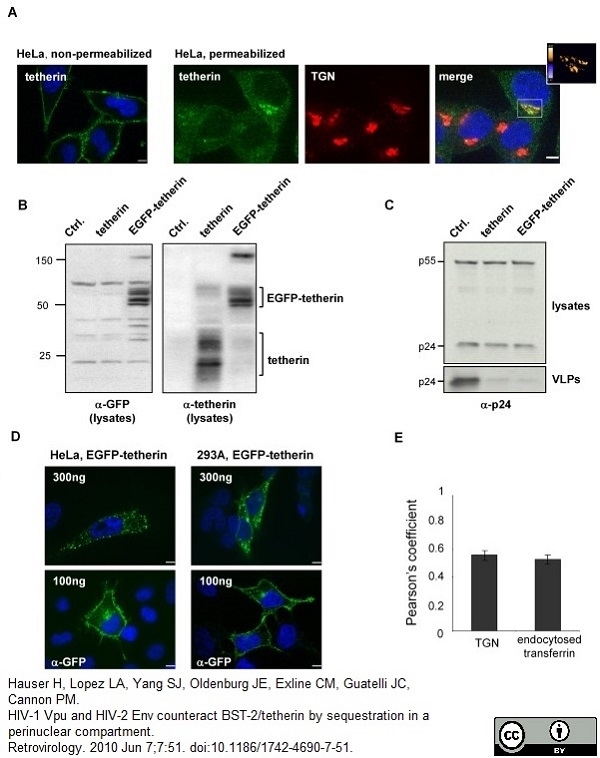
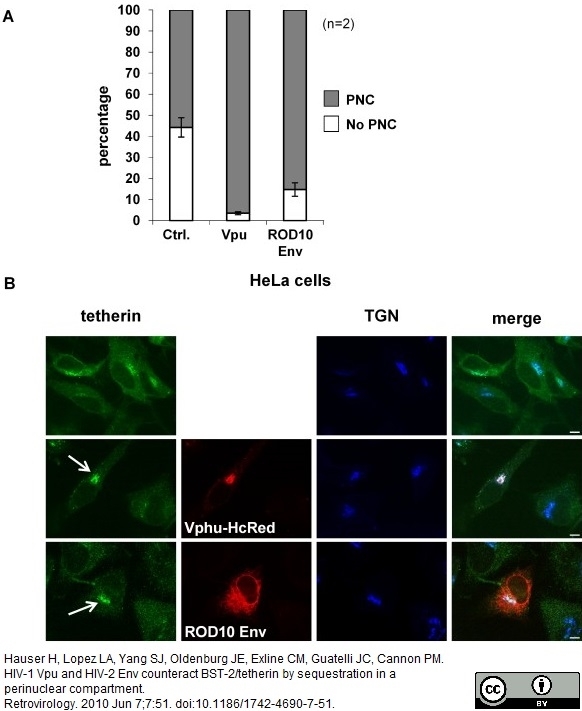
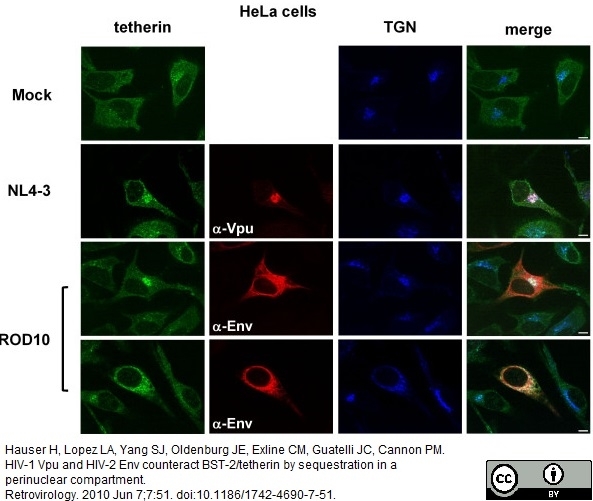
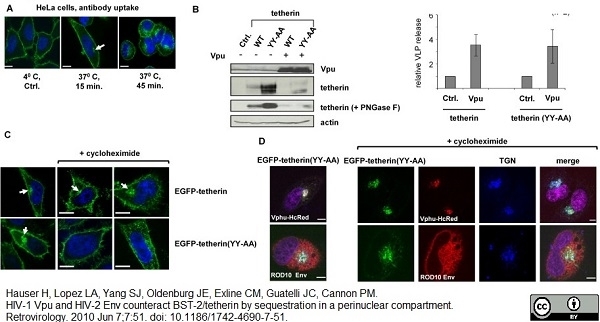
Hauser, H. et al. (2010) HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment.
Retrovirology. 7: 51. -
Roberts, R.C. et al. (2010) Mistargeting of SH3TC2 away from the recycling endosome causes Charcot-Marie-Tooth disease type 4C.
Hum Mol Genet.19: 1009-18. -
Fairn, G.D. et al. (2011) High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine.
J Cell Biol. 194 (2): 257-75. -
Vleck, S.E. et al. (2011) Structure-function analysis of varicella-zoster virus glycoprotein H identifies domain-specific roles for fusion and skin tropism.
Proc Natl Acad Sci U S A. 108 (45): 18412-7. -
Oliver, S.L. et al. (2011) Mutagenesis of varicella-zoster virus glycoprotein I (gI) identifies a cysteine residue critical for gE/gI heterodimer formation, gI structure, and virulence in skin cells.
J Virol. 85 (9): 4095-110. -
Petit, S.J. et al. (2011) Analysis of the human immunodeficiency virus type 1 M group Vpu domains involved in antagonizing tetherin.
J Gen Virol. 92 (Pt 12): 2937-48. -
Zuckerman, D.M. et al. (2011) Differential regulation of two palmitoylation sites in the cytoplasmic tail of the beta1-adrenergic receptor.
J Biol Chem. 286: 19014-23. -
Laufman, O. et al. (2011) The COG complex interacts directly with Syntaxin 6 and positively regulates endosome-to-TGN retrograde transport.
J Cell Biol. 194 (3): 459-72. -
Uchida, Y. et al. (2011) Intracellular phosphatidylserine is essential for retrograde membrane traffic through endosomes.
Proc Natl Acad Sci U S A. 108 (38): 15846-51. -
Cheng, S.B. et al. (2011) Down-modulation of the G-protein-coupled Estrogen Receptor, GPER, from the Cell Surface Occurs via a trans-Golgi-Proteasome Pathway.
J Biol Chem. 286: 22441-55. -
Kwon, S. and Christian, J.L. (2011) Sortilin Associates with Transforming Growth Factor-{beta} Family Proteins to Enhance Lysosome-mediated Degradation.
J Biol Chem. 286: 21876-85. -
Kawabata, A. et al. (2011) Analysis of a Neutralizing Antibody for Human Herpesvirus 6B Reveals a Role for Glycoprotein Q1 in Viral Entry.
J Virol. 85: 12962-71. -
Cornfine, S. et al. (2011) The kinesin KIF9 and reggie/flotillin proteins regulate matrix degradation by macrophage podosomes.
Mol Biol Cell. 22: 202-15. -
Vorobyeva, A.G. et al. (2014) Cyclopamine modulates γ-secretase-mediated cleavage of amyloid precursor protein by altering its subcellular trafficking and lysosomal degradation.
J Biol Chem. 289 (48): 33258-74. -
Chia, R. et al. (2014) Phosphorylation of LRRK2 by casein kinase 1α regulates trans-Golgi clustering via differential interaction with ARHGEF7.
Nat Commun. 5: 5827. -
Wang Z et al. (2014) A newly identified myomegalin isoform functions in Golgi microtubule organization and ER-Golgi transport.
J Cell Sci. 127 (22): 4904-17. -
DiGiuseppe, S. et al. (2015) Topography of the Human Papillomavirus Minor Capsid Protein L2 during Vesicular Trafficking of Infectious Entry.
J Virol. 89 (20): 10442-52. -
Gottschalk, E.Y. & Meneses, P.I. (2015) A Dual Role for the Nonreceptor Tyrosine Kinase Pyk2 during the Intracellular Trafficking of Human Papillomavirus 16.
J Virol. 89 (17): 9103-14. -
Ioannou, M.S. et al. (2015) DENND2B activates Rab13 at the leading edge of migrating cells and promotes metastatic behavior.
J Cell Biol. 208 (5): 629-48. -
El Kasmi, I. & Lippé, R. (2015) Herpes simplex virus 1 gN partners with gM to modulate the viral fusion machinery.
J Virol. 89 (4): 2313-23. -
Luo, S. et al. (2015) Contribution of N-linked glycans on HSV-2 gB to cell-cell fusion and viral entry.
Virology. 483: 72-82. -
Ikawa Y et al. (2015) In vitro functional correction of Hermansky-Pudlak Syndrome type-1 by lentiviral-mediated gene transfer.
Mol Genet Metab. 114 (1): 62-5. -
Crevenna, A.H. et al. (2016) Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network.
J Cell Biol. 213 (3): 305-14. -
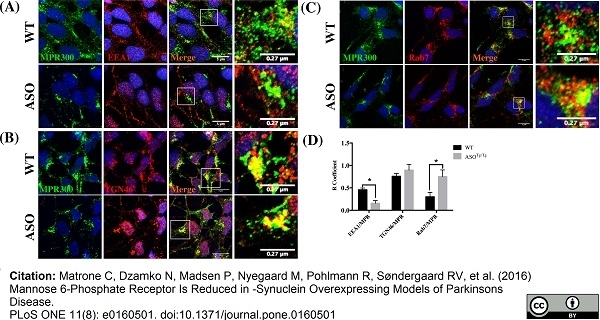
Matrone, C. et al. (2016) Mannose 6-Phosphate Receptor Is Reduced in -Synuclein Overexpressing Models of Parkinsons Disease.
PLoS One. 11 (8): e0160501. -
Haugsten, E.M. et al. (2016) Proximity Labeling Reveals Molecular Determinants of FGFR4 Endosomal Transport.
J Proteome Res. 15 (10): 3841-55. -
Paquin, N. et al. (2016) The Conserved VPS-50 Protein Functions in Dense-Core Vesicle Maturation and Acidification and Controls Animal Behavior.
Curr Biol. 26 (7): 862-71. -
Ketteler, R. et al. (2017) Image-based siRNA screen to identify kinases regulating Weibel-Palade body size control using electroporation.
Sci Data. 4: 170022. -
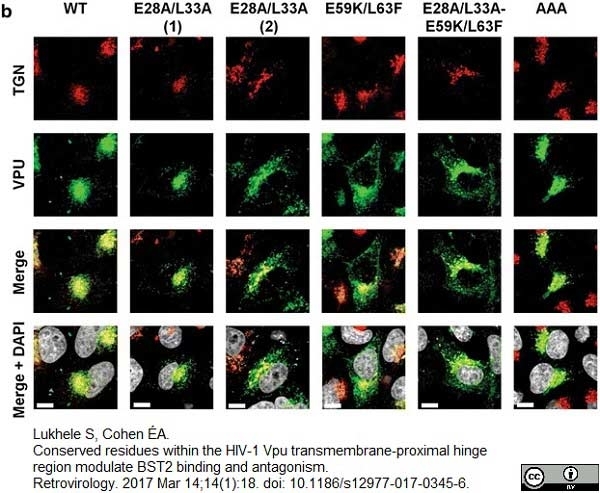
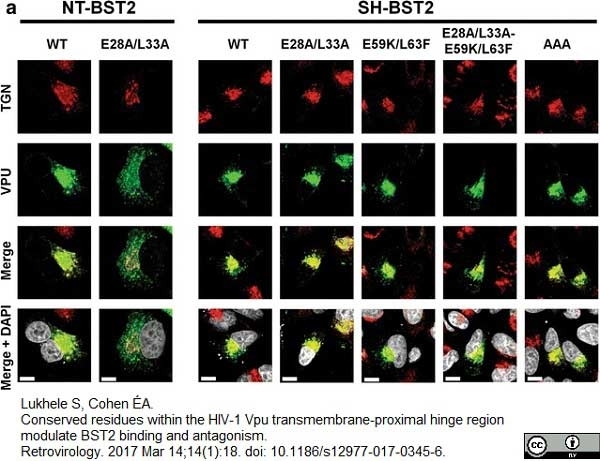
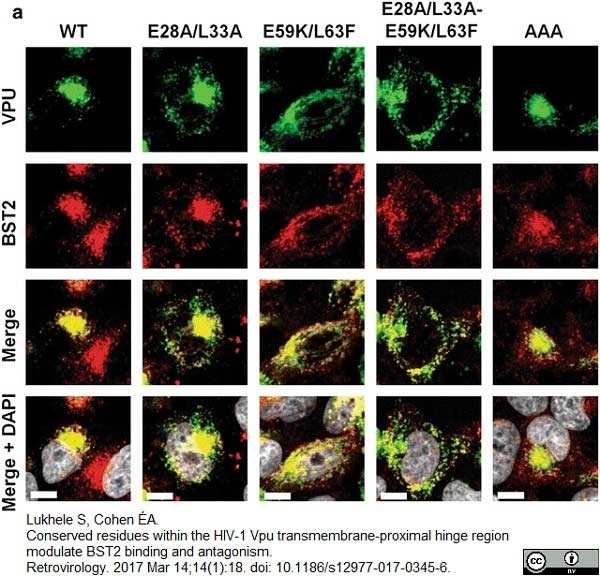
Lukhele, S. & Cohen É.A. (2017) Conserved residues within the HIV-1 Vpu transmembrane-proximal hinge region modulate BST2 binding and antagonism.
Retrovirology. 14 (1): 18. -
Sugden, S.M. et al. (2017) HIV-1 Vpu Downmodulates ICAM-1 Expression, Resulting in Decreased Killing of Infected CD4+ T Cells by NK Cells.
J Virol. 91 (8): pii: e02442-16. -
Cabukusta, B. et al. (2017) Ceramide phosphoethanolamine synthase SMSr is a target of Caspase-6 during apoptotic cell death.
Biosci Rep. 37 (4): BSR20170867. -
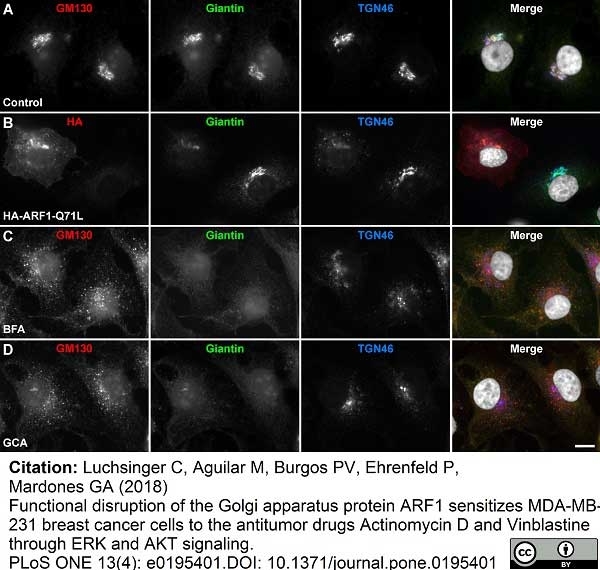
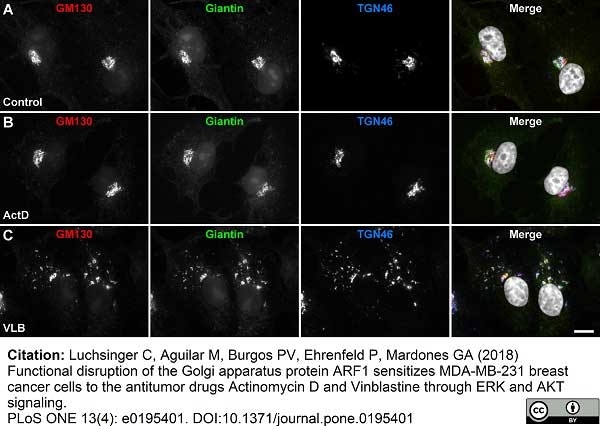
Luchsinger, C. et al. (2018) Functional disruption of the Golgi apparatus protein ARF1 sensitizes MDA-MB-231 breast cancer cells to the antitumor drugs Actinomycin D and Vinblastine through ERK and AKT signaling.
PLoS One. 13 (4): e0195401. -
Ayala, I. et al. (2019) GRASP65 controls Golgi position and structure during G2/M transition by regulating the stability of microtubules.
Traffic. 20 (10): 785-802. -
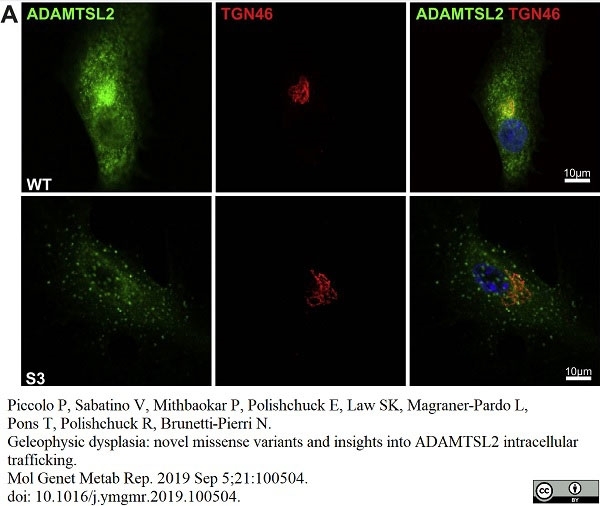
Piccolo, P. et al. (2019) Geleophysic dysplasia: novel missense variants and insights into ADAMTSL2 intracellular trafficking.
Mol Genet Metab Rep. 21: 100504. -
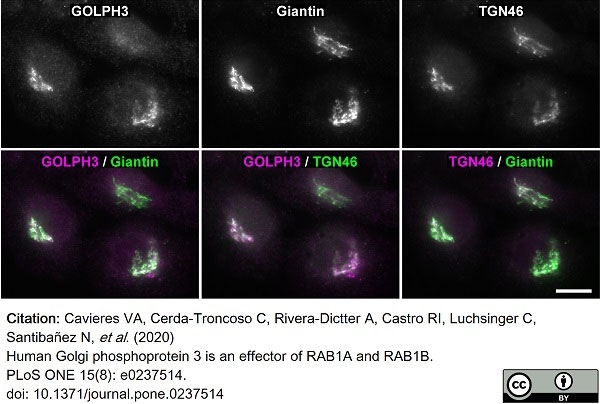
Cavieres, V.A. et al. (2020) Human Golgi phosphoprotein 3 is an effector of RAB1A and RAB1B.
PLoS One. 15 (8): e0237514. -
Sakuma, C. et al. (2021) Identification of SYS1 as a Host Factor Required for Shiga Toxin-Mediated Cytotoxicity in Vero Cells
Int J Mol Sci 22 (9): 4936. -
Besemer, A.S. et al. (2021) Receptor-mediated endocytosis 8 (RME-8)/DNAJC13 is a novel positive modulator of autophagy and stabilizes cellular protein homeostasis.
Cell Mol Life Sci. 78 (2): 645-60. -
Stoneham, C.A. et al. (2021) A combined EM and proteomic analysis places HIV-1 Vpu at the crossroads of retromer and ESCRT complexes: PTPN23 is a Vpu-cofactor.
PLoS Pathog. 17 (11): e1009409. -
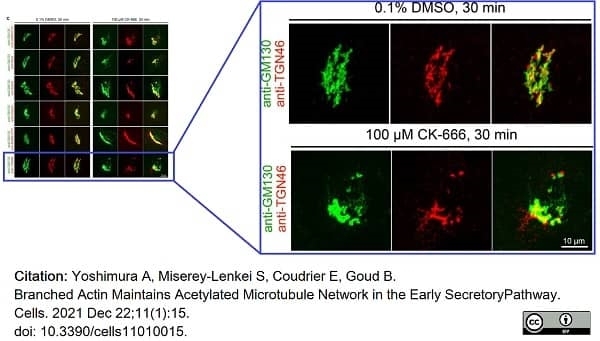
Yoshimura, A. et al. (2021) Branched Actin Maintains Acetylated Microtubule Network in the Early Secretory Pathway
Cells. 11 (1): 15. -
Bracci, N. et al. (2022) Rift Valley fever virus Gn V5-epitope tagged virus enables identification of UBR4 as a Gn interacting protein that facilitates Rift Valley fever virus production.
Virology. 567: 65-76. -
Zhang, J. et al. (2022) SARS-CoV-2 triggers Golgi fragmentation via down-regulation of GRASP55 to facilitate viral trafficking [Preprint]
bioRxiv 09 Mar [Epub ahead of print]. -
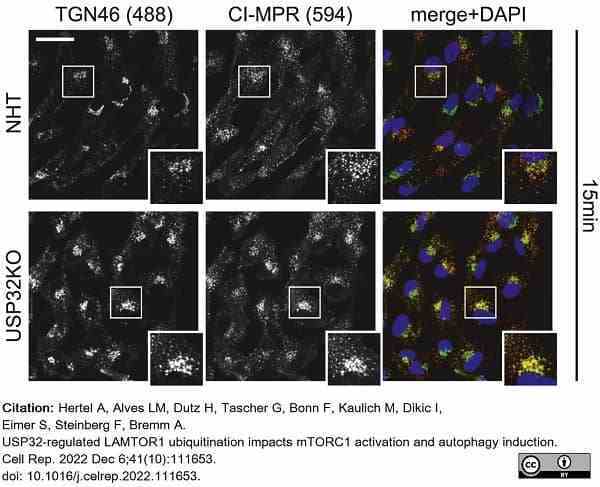
Hertel, A. et al. (2022) USP32-regulated LAMTOR1 ubiquitination impacts mTORC1 activation and autophagy induction.
Cell Rep. 41 (10): 111653. -
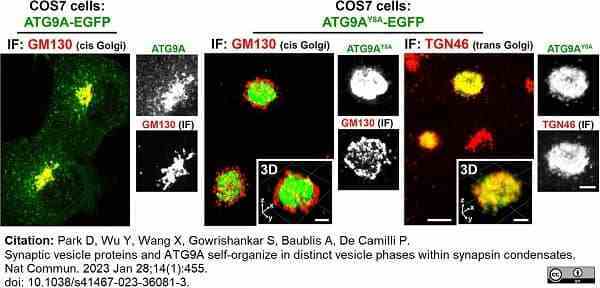
Park, D. et al. (2023) Synaptic vesicle proteins and ATG9A self-organize in distinct vesicle phases within synapsin condensates.
Nat Commun. 14 (1): 455. -
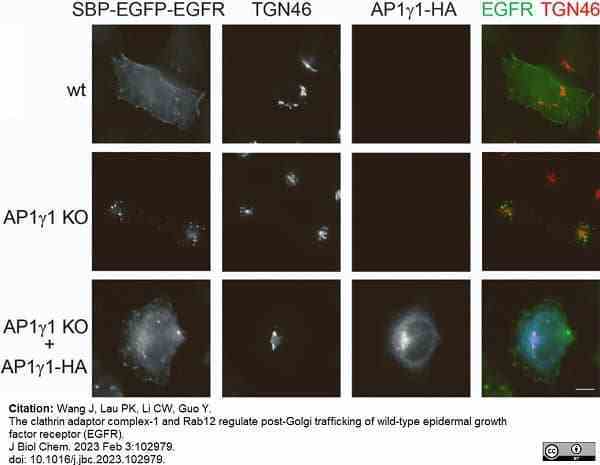
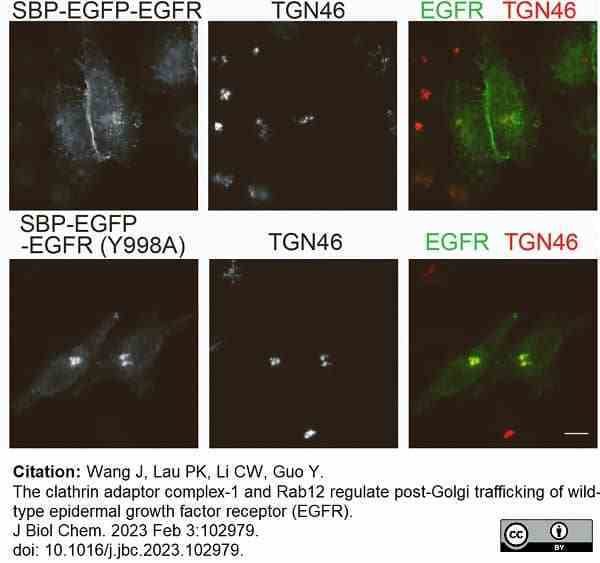
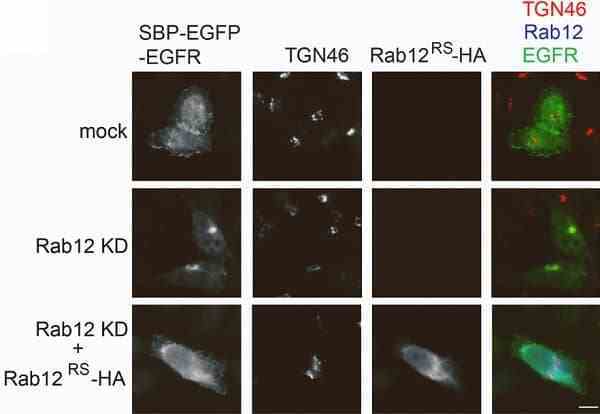
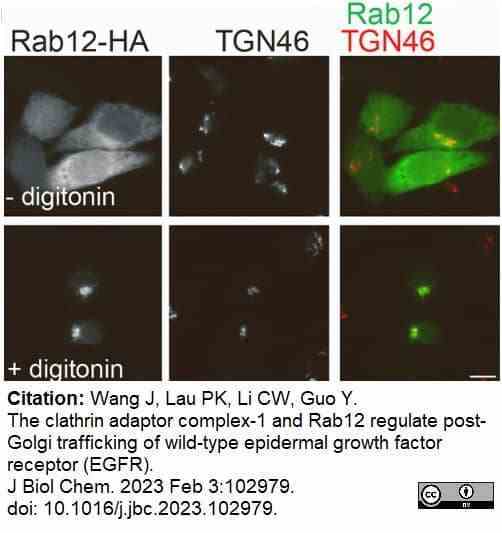
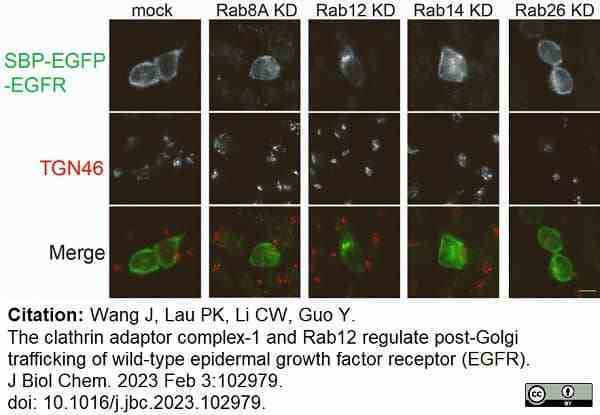
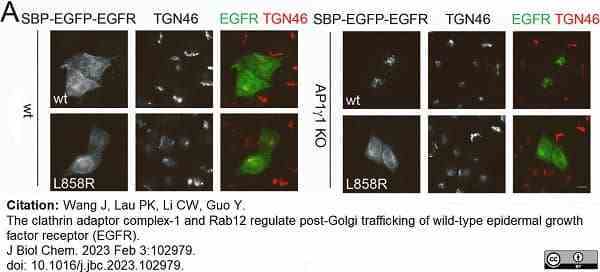
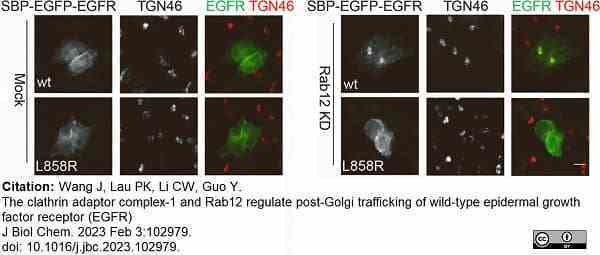
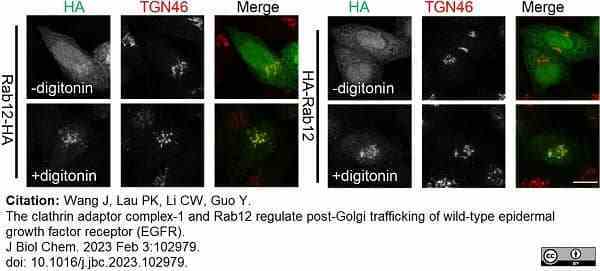
Wang, J. et al. (2023) The clathrin adaptor complex-1 and Rab12 regulate post-Golgi trafficking of wild-type epidermal growth factor receptor (EGFR).
J Biol Chem. : 102979. -
Nishino, M. et al. (2023) Histone methyltransferase SUV39H1 regulates the Golgi complex via the nuclear envelope-spanning LINC complex.
PLoS One. 18 (7): e0283490. -
Hao, H. et al. (2020) Golgi-associated microtubules are fast cargo tracks and required for persistent cell migration.
EMBO Rep. 21 (3): e48385. -
Fujimoto, T. et al. (2018) Parkinson's disease-associated mutant LRRK2 phosphorylates Rab7L1 and modifies trans-Golgi morphology.
Biochem Biophys Res Commun. 495 (2): 1708-15. -
Venditti, R. et al. (2019) Molecular determinants of ER-Golgi contacts identified through a new FRET-FLIM system.
J Cell Biol. 218 (3): 1055-65. -
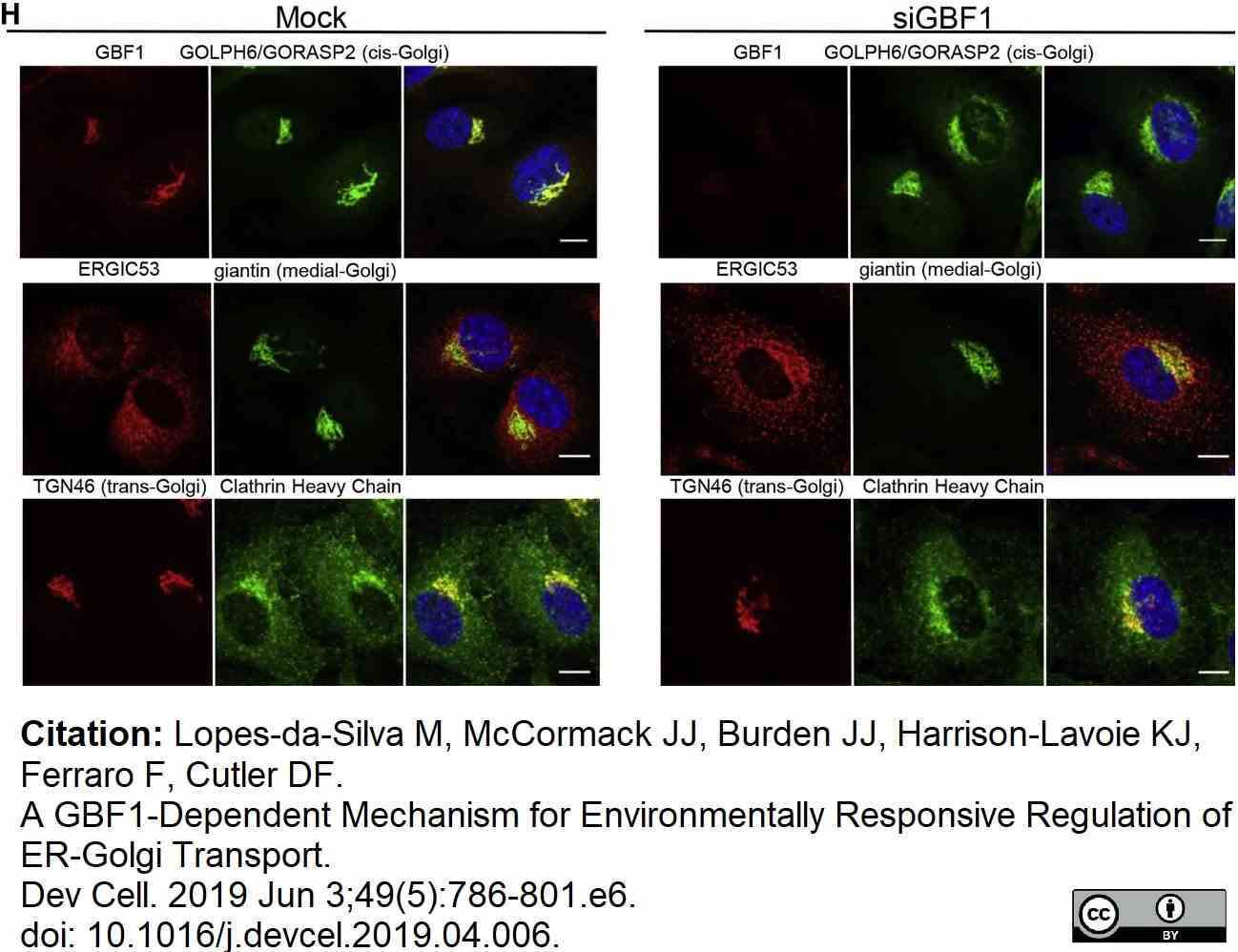
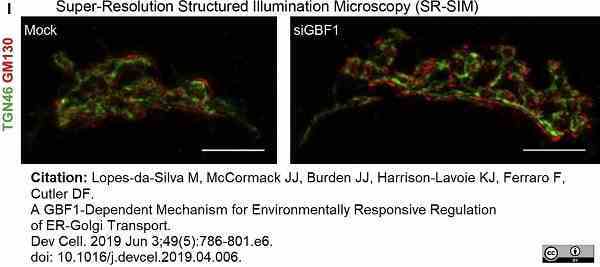
Lopes-da-Silva, M. et al. (2019) A GBF1-Dependent Mechanism for Environmentally Responsive Regulation of ER-Golgi Transport.
Dev Cell. 49 (5): 786-801.e6. -
Wakana, Y. et al. (2021) The ER cholesterol sensor SCAP promotes CARTS biogenesis at ER-Golgi membrane contact sites.
J Cell Biol. 220 (1): e202002150. -
Hieda, M. et al. (2021) The SUN2-nesprin-2 LINC complex and KIF20A function in the Golgi dispersal.
Sci Rep. 11 (1): 5358. -
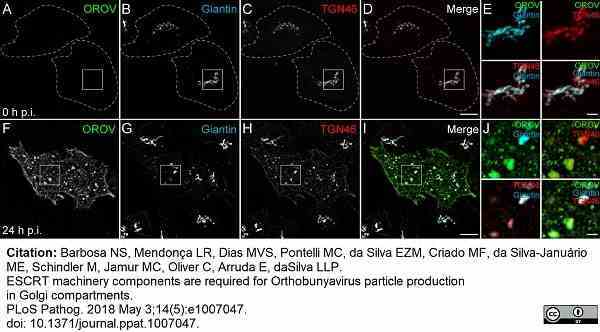
Barbosa, N.S. et al. (2018) ESCRT machinery components are required for Orthobunyavirus particle production in Golgi compartments.
PLoS Pathog. 14 (5): e1007047. -
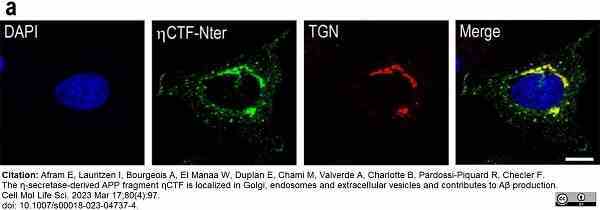
Afram, E. et al. (2023) The η-secretase-derived APP fragment ηCTF is localized in Golgi, endosomes and extracellular vesicles and contributes to Aβ production.
Cell Mol Life Sci. 80 (4): 97. -
van der Beek, J. et al. (2024) Loss of the HOPS complex disrupts early-to-late endosome transition, impairs endosomal recycling and induces accumulation of amphisomes.
Mol Biol Cell. : mbcE23080328 [Epub ahead of print] -
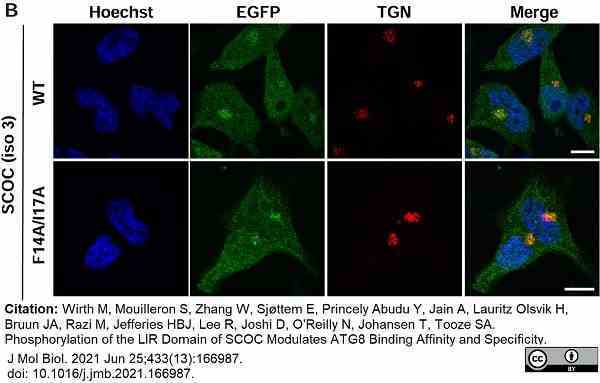
Wirth, M. et al. (2021) Phosphorylation of the LIR Domain of SCOC Modulates ATG8 Binding Affinity and Specificity.
J Mol Biol. 433 (13): 166987. -
Hecht, T.K. et al. (2020) Fam20C regulates protein secretion by Cab45 phosphorylation.
J Cell Biol. 219 (6): e201910089. -
Jermusyk, A. et al. (2021) A 584 bp deletion in CTRB2 inhibits chymotrypsin B2 activity and secretion and confers risk of pancreatic cancer.
Am J Hum Genet. 108 (10): 1852-65. -
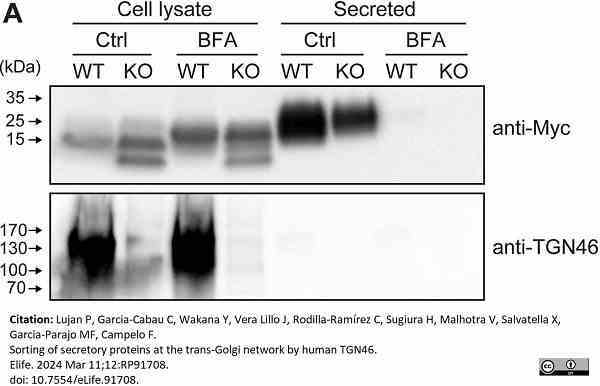
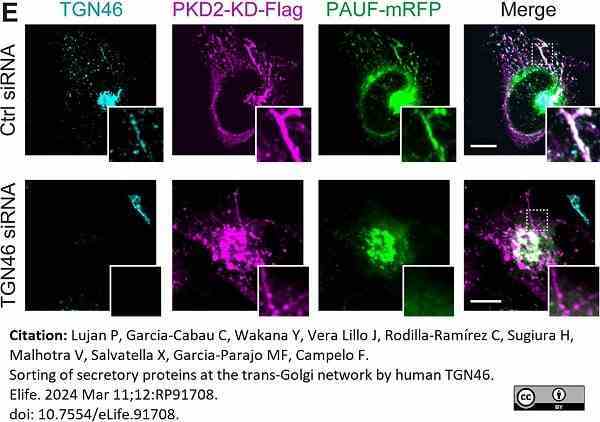
Lujan, P. et al. (2024) Sorting of secretory proteins at the trans-Golgi network by human TGN46.
Elife. 12: RP91708.
Further Reading
-
Ponnambalam, S. et al. (1996) Primate homologues of rat TGN38: primary structure, expression and functional implications.
J Cell Sci. 109 ( Pt 3): 675-85.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Human ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up