APC Conjugation Kit

LYNX Rapid APC Antibody Conjugation Kit
- Product Type
- Conjugation Kit
- Specificity
- APC Conjugation Kit
| Product Code | Applications | Pack Size | List Price | Your Price | Qty | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| LYNX Rapid APC Antibody Conjugation Kit® enables the rapid conjugation of a pre-prepared lyophilized mixture containing Allophycocyanin (APC) label to an antibody or protein. Activation of proprietary reagents within the antibody-label solution results in directional covalent bonding of APC to the antibody. The LYNX Rapid Conjugation kit® can be used to label small quantities of antibody/protein at near neutral pH, allowing a high conjugation efficiency with 100% antibody recovery. |
- Reagents In The Kit
-
LNK034APC: 3 Vials of 10ug LYNX lyophilized APC mix
1 Vial LYNX Modifier reagent
1 Vial LYNX Quencher reagent
-
LNK033APC: 1 Vial of 1mg LYNX lyophilized APC mix
1 Vial LYNX Modifier reagent
1 Vial LYNX Quencher reagent
-
LNK032APC: 3 Vials of 100ug LYNX lyophilized APC mix
1 Vial LYNX Modifier reagent
1 Vial LYNX Quencher reagent
-
LNK031APC: 1 Vial of 100ug LYNX lyophilized APC mix
1 Vial LYNX Modifier reagent
1 Vial LYNX Quencher reagent
- Preparing The Antibody
-
LNK034APC: The following buffer solutions are recommended for preparing the antibody:
10-50mM amine-free buffer (e.g HEPES, MES, MOPS and phosphate) pH range 6.5-8.5, although moderate concentrations of Tris buffer (<20mM) may be tolerated.
If possible, avoid buffers containing nucleophilic components such as primary amines and thiols (e.g. thiomersal/thimerosal) since they may react with LYNX chemicals. Azide (0.02-0.1%), EDTA, up to 50% Glycerol and common non-buffering salts and sugars have little or no effect on conjugation efficiency.
For optimal results the antibody should be at a concentration of 1mg/ml, with a maximum volume of 10ul and a maximum antibody amount of 10ug. Antibody at a concentration of greater than 1mg/ml requires dilution. Antibody below 1mg/ml can still be used as long as the maximum volume is not exceeded. Using less than the recommended amount of antibody may result in unbound label, but this will be removed during subsequent application wash steps. Antibody below 0.5mg/ml should be concentrated before use with the kit. -
LNK033APC: The following buffer solutions are recommended for preparing the antibody:
10-50mM amine-free buffer (e.g HEPES, MES, MOPS and phosphate) pH range 6.5-8.5, although moderate concentrations of Tris buffer (<20mM) may be tolerated.
If possible, avoid buffers containing nucleophilic components such as primary amines and thiols (e.g. thiomersal/thimerosal) since they may react with LYNX chemicals. Azide (0.02-0.1%), EDTA, up to 50% Glycerol and common non-buffering salts and sugars have little or no effect on conjugation efficiency.
For optimal results the antibody should be at a concentration of 1mg/ml, with a maximum volume of 1ml and a maximum antibody amount of 1mg. Antibody at a concentration of greater than 1mg/ml requires dilution. Antibody below 1mg/ml can still be used as long as the maximum volume is not exceeded. Using less than the recommended amount of antibody may result in unbound label, but this will be removed during subsequent application wash steps. Antibody below 0.5mg/ml should be concentrated before use with the kit. -
LNK032APC, LNK031APC: The following buffer solutions are recommended for preparing the antibody:
10-50mM amine-free buffer (e.g HEPES, MES, MOPS and phosphate) pH range 6.5-8.5, although moderate concentrations of Tris buffer (<20mM) may be tolerated.
If possible, avoid buffers containing nucleophilic components such as primary amines and thiols (e.g. thiomersal/thimerosal) since they may react with LYNX chemicals. Azide (0.02-0.1%), EDTA, up to 50% Glycerol and common non-buffering salts and sugars have little or no effect on conjugation efficiency.
For optimal results the antibody should be at a concentration of 1mg/ml, with a maximum volume of 100ul and a maximum antibody amount of 100ug. Antibody at a concentration of greater than 1mg/ml requires dilution. Antibody below 1mg/ml can still be used as long as the maximum volume is not exceeded. Using less than the recommended amount of antibody may result in unbound label, but this will be removed during subsequent application wash steps. Antibody below 0.5mg/ml should be concentrated before use with the kit. - Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Conjugation |
We recommend that for each conjugation the user determines the best antibody:conjugate ratio.
- Instructions For Use
- 1. To the antibody sample add 1ul of the Modifier reagent for every 10ul of antibody and mix gently.
2. Pipette the mixed antibody-modifier sample directly onto the LYNX lyophilized mix and gently pipette up and down twice to resuspend.
3. Replace cap onto vial and incubate in the dark at room temperature (20-25oC) for 3 hours, or overnight if preferred.
4. After incubation, add 1ul of Quencher reagent for every 10ul of antibody used. Leave to stand for 30 minutes before use. - 1. To the antibody sample add 1ul of the Modifier reagent for every 10ul of antibody and mix gently.
2. Pipette the mixed antibody-modifier sample directly onto the LYNX lyophilized mix and gently pipette up and down twice to resuspend.
3. Replace cap onto vial and incubate in the dark at room temperature (20-25oC) for 3 hours, or overnight if preferred.
4. After incubation, add 1ul of Quencher reagent for every 10ul of antibody used. Leave to stand for 30 minutes before use. - 1. To the antibody sample add 1ul of the Modifier reagent for every 10ul of antibody and mix gently.
2. Pipette the mixed antibody-modifier sample directly onto the LYNX lyophilized mix and gently pipette up and down twice to resuspend.
3. Replace cap onto vial and incubate in the dark at room temperature (20-25oC) for 3 hours, or overnight if preferred.
4. After incubation, add 1ul of Quencher reagent for every 10ul of antibody used. Leave to stand for 30 minutes before use. - 1. To the antibody sample add 1ul of the Modifier reagent for every 10ul of antibody and mix gently.
2. Pipette the mixed antibody-modifier sample directly onto the LYNX lyophilized mix and gently pipette up and down twice to resuspend.
3. Replace cap onto vial and incubate in the dark at room temperature (20-25oC) for 3 hours, or overnight if preferred.
4. After incubation, add 1ul of Quencher reagent for every 10ul of antibody used. Leave to stand for 30 minutes before use.
References for APC Conjugation Kit
-
Wang, Y. et al. (2010) Local host response to chlamydial urethral infection in male guinea pigs.
Infect Immun.78: 1670-81. -
Lacy, H.M. et al. (2011) Essential Role for Neutrophils in Pathogenesis and Adaptive Immunity in Chlamydia caviae Ocular Infections.
Infect Immun. 79: 1889-97 -
Paget, C. et al. (2012) Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damage.
J Biol Chem. 287: 8816-29. -
Seliger, C. et al. (2011) A rapid high-precision flow cytometry based technique for total white blood cell counting in chickens.
Vet Immunol Immunopathol. 145: 86-99. -
Fu, Y. et al. (2014) Development of a FACS-based assay for evaluating antiviral potency of compound in dengue infected peripheral blood mononuclear cells.
J Virol Methods. 196: 18-24. -
Traxlmayr, M.W. et al. (2014) Construction of pH-sensitive Her2-binding IgG1-Fc by directed evolution.
Biotechnol J. 9: 1013-22. -
Wielgosz, M.M. et al. (2015) Generation of a lentiviral vector producer cell clone for human Wiskott-Aldrich syndrome gene therapy.
Mol Ther Methods Clin Dev. 2: 14063. -
Hofer, C.C. et al. (2015) Infection of mice with influenza A/WSN/33 (H1N1) virus alters alveolar type II cell phenotype.
Am J Physiol Lung Cell Mol Physiol. 308 (7): L628-38.
View The Latest Product References
-
Poh, C.M. et al. (2014) Damage to the blood-brain barrier during experimental cerebral malaria results from synergistic effects of CD8+ T cells with different specificities.
Infect Immun. 82: 4854-64. -
Hasenhindl, C. et al. (2014) Creating stable stem regions for loop elongation in Fcabs - insights from combining yeast surface display, in silico loop reconstruction and molecular dynamics simulations.
Biochim Biophys Acta.1844: 1530-40. -
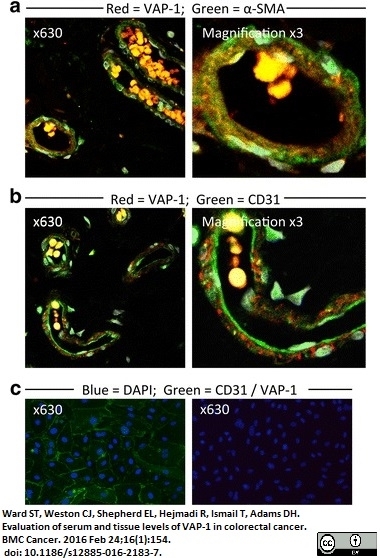
Ward, S.T. et al. (2016) Evaluation of serum and tissue levels of VAP-1 in colorectal cancer.
BMC Cancer. 16 (1): 154. -
Schuh, C.M. et al. (2016) Covalent binding of placental derived proteins to silk fibroin improves schwann cell adhesion and proliferation.
J Mater Sci Mater Med. 27 (12): 188. -
Hercher, D. et al. (2020) Motor and sensory Schwann cell phenotype commitment is diminished by extracorporeal shockwave treatment in vitro..
J Peripher Nerv Syst. 25 (1): 32-43. -
Theuerkauf, K. et al. (2022) Activated platelets and platelet-leukocyte aggregates in the equine systemic inflammatory response syndrome.
J Vet Diagn Invest. : 10406387221077969. -
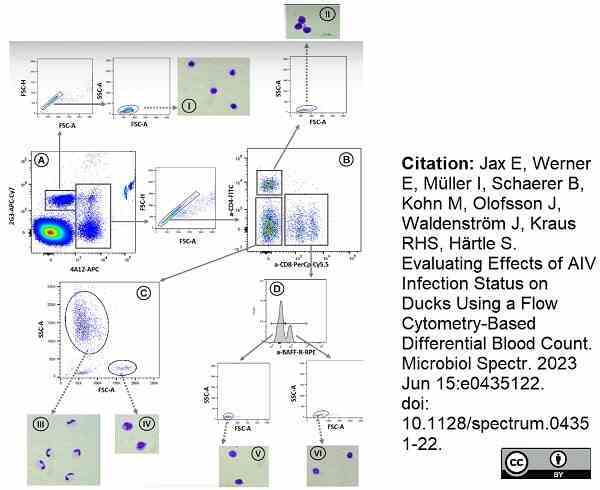
Jax, E. et al. (2023) Evaluating Effects of AIV Infection Status on Ducks Using a Flow Cytometry-Based Differential Blood Count.
Microbiol Spectr. 11 (4): e0435122. -
Haach, V. et al. (2023) A polyvalent virosomal influenza vaccine induces broad cellular and humoral immunity in pigs.
Virol J. 20 (1): 181.
- Licensed Use
- These products and the methodology of conjugation are patent protected under United Kingdom patent number 2446088 and associated international patent applications. The purchase of this product conveys to the buyer the limited, non exclusive non-transferable right (without the right to resell repackage or further sublicense) under these patents to use the product to make conjugates for research and development purposes only. The purchaser cannot sell or otherwise transfer this product, or its components, or materials or data made using this product, or its components to a third party. Further information on purchasing licenses for diagnostic and other uses may be obtained by contacting Bio-Rad, at. Endeavour House, Langford Business Park, Langford Lane, Kidlington, Oxon. OX5 1GE UNITED KINGDOM. Tel: +44 1865 852 700. E-mail: antibodies@bio-rad.com
LNK034APC
LNK033APC
LNK032APC
LNK031APC
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
Always be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up