Baby Rabbit Complement




Baby Rabbit Complement
- Product Type
- Serum
- Specificity
- Baby Rabbit Complement
| Baby rabbit complement serum preparation is intended for use as a source of rabbit complement for cytotoxicity assays. |
- Product Form
- Baby rabbit serum - lyophilized
- Reconstitution
- C12CA.1: Reconstitute with 1.0 ml ice cold distilled water
- C12CA: Reconstitute with 2ml ice cold distilled water
- Preservative Stabilisers
- None present
- Regulatory
- For research purposes only
- Guarantee
- Guaranteed until date of expiry. Please see product label.
This product should be stored undiluted. Avoid repeated freezing and thawing as this may denature the product. Should this product contain a precipitate we recommend microcentrifugation before use.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Functional Assays 1 | |||
| Immunoassay |
- 1 This product is not sold as sterile but can be sterilized by filtration if necessary. It is preferable to dilute the complement to a final working concentration before filtration in order to minimize loss of volume.
- Instructions For Use
- Use within one hour of reconstitution, keeping on ice throughout.
References for Baby Rabbit Complement
-
De clercq, L. et al. (1997) An anti-adipocyte monoclonal antibody is cytotoxic to porcine preadipocytes in vitro and depresses the development of pig adipose tissue.
J Anim Sci. 75 (7): 1791-7. -
Anderson, L.D. Jr et al. (1999) Enhancement of graft-versus-tumor activity and graft-versus-host disease by pretransplant immunization of allogeneic bone marrow donors with a recipient-derived tumor cell vaccine.
Cancer Res. 59 (7): 1525-30. -
Lidington, E.A. et al. (2000) Induction of decay-accelerating factor by thrombin through a protease-activated receptor 1 and protein kinase C-dependent pathway protects vascular endothelial cells from complement-mediated injury.
Blood. 96 (8): 2784-92. -
Mason, J.C. et al. (2002) bFGF and VEGF synergistically enhance endothelial cytoprotection via decay-accelerating factor induction.
Am J Physiol Cell Physiol. 282: C578-87. -
Mason, J.C. et al. (2002) Statin-induced expression of decay-accelerating factor protects vascular endothelium against complement-mediated injury.
Circ Res. 91 (8): 696-703. -
Li, S.H. et al. (2004) C-reactive protein upregulates complement-inhibitory factors in endothelial cells.
Circulation. 109: 833-6. -
Newcombe, J. et al. (2004) Infection with an avirulent phoP mutant of Neisseria meningitidis confers broad cross-reactive immunity.
Infect Immun. 72: 338-44. -
Sancho, D. et al. (2006) CD69 targeting differentially affects the course of collagen-induced arthritis.
J Leukoc Biol. 80: 1233-41.
View The Latest Product References
-
Hyams, C. et al. (2010) Streptococcus pneumoniae resistance to complement-mediated immunity is dependent on the capsular serotype.
Infect Immun. 78: 716-25. -
Hung, M.C. et al. (2011) The Neisseria meningitidis Macrophage Infectivity Potentiator Protein Induces Cross-Strain Serum Bactericidal Activity and Is a Potential Serogroup B Vaccine Candidate.
Infect Immun. 79: 3784-91. -
Lee, S.J. et al. (2012) Identification of a common immune signature in murine and human systemic Salmonellosis.
Proc Natl Acad Sci U S A. 109 (13): 4998-5003. -
Hung MC et al. (2013) The adhesin complex protein (ACP) of Neisseria meningitidis is a new adhesin with vaccine potential.
MBio. 4 (2): pii: e00041-13. -
Goh, Y.S. & MacLennan, C.A. (2013) Invasive African nontyphoidal Salmonella requires high levels of complement for cell-free antibody-dependent killing.
J Immunol Methods. 387 (1-2): 121-9. -
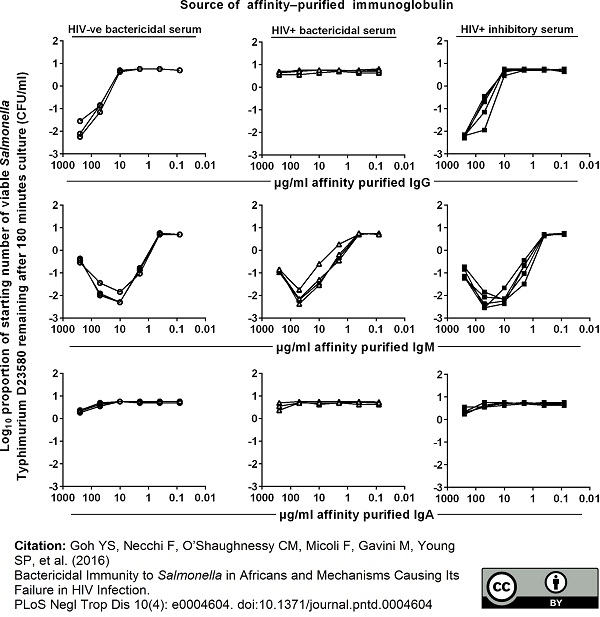
Goh YS et al. (2016) Bactericidal Immunity to Salmonella in Africans and Mechanisms Causing Its Failure in HIV Infection.
PLoS Negl Trop Dis. 10 (4): e0004604. -
Humbert MV et al. (2016) Vaccine Potential and Diversity of the Putative Cell Binding Factor (CBF, NMB0345/NEIS1825) Protein of Neisseria meningitidis.
PLoS One. 11 (8): e0160403. -
Dierckx de Casterlé I et al. (2018) Reduction of myeloid-derived suppressor cells reinforces the anti-solid tumor effect of recipient leukocyte infusion in murine neuroblastoma-bearing allogeneic bone marrow chimeras.
Cancer Immunol Immunother. 67 (4): 589-603. -
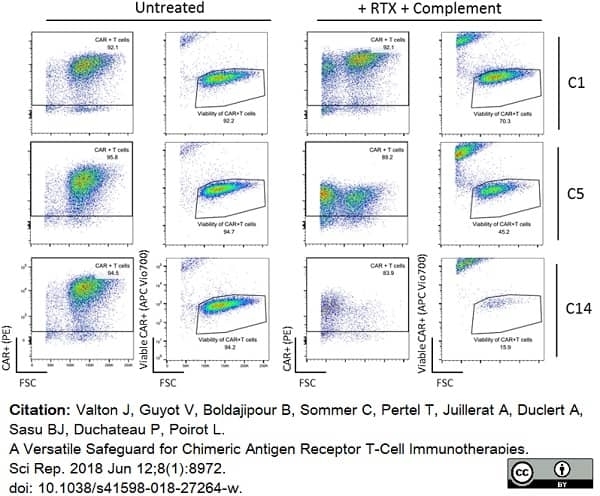
Valton, J. et al. (2018) A Versatile Safeguard for Chimeric Antigen Receptor T-Cell Immunotherapies.
Sci Rep. 8 (1): 8972. -
Dierckx de Casterlé, I. et al. (2018) Reduction of myeloid-derived suppressor cells reinforces the anti-solid tumor effect of recipient leukocyte infusion in murine neuroblastoma-bearing allogeneic bone marrow chimeras.
Cancer Immunol Immunother. 67 (4): 589-603. -
Nganje, C.N. et al. (2019) PepN is a non-essential, cell wall-localized protein that contributes to neutrophil elastase-mediated killing of Streptococcus pneumoniae.
PLoS One. 14 (2): e0211632. -
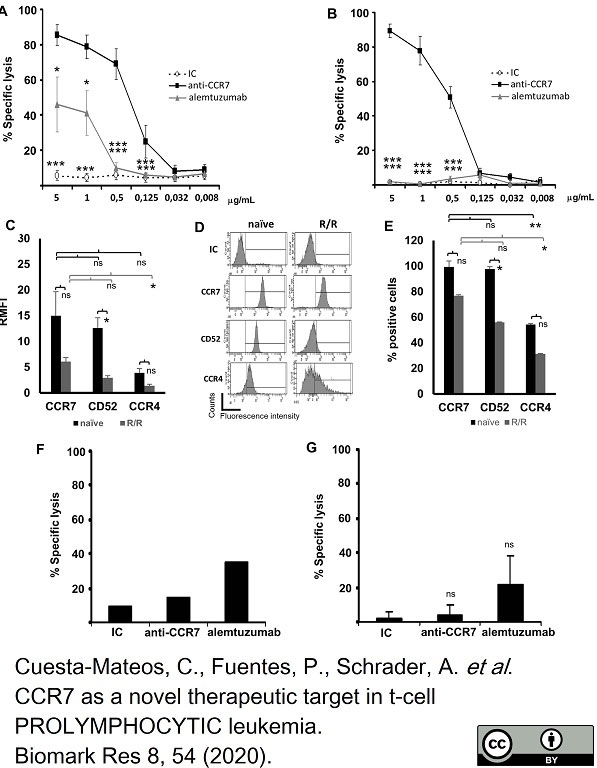
Cuesta-Mateos, C. et al. (2020) CCR7 as a novel therapeutic target in t-cell PROLYMPHOCYTIC leukemia
Biomarker Research.8, 54. -
Mosti, L. et al. (2021) Targeted multi-epitope switching enables straightforward positive/negative selection of CAR T cells.
Gene Ther. 28 (9): 602-12. -
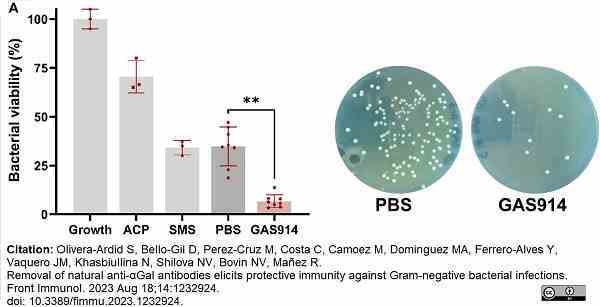
Olivera-Ardid, S. et al. (2023) Removal of natural anti-αGal antibodies elicits protective immunity against Gram-negative bacterial infections.
Front Immunol. 14: 1232924.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
Always be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up