VIVAFIX™ Assay


VIVAFIX™ 353/442 Cell Viability Assay
- Product Type
- Accessory Reagent
- Specificity
- VIVAFIX™ Assay
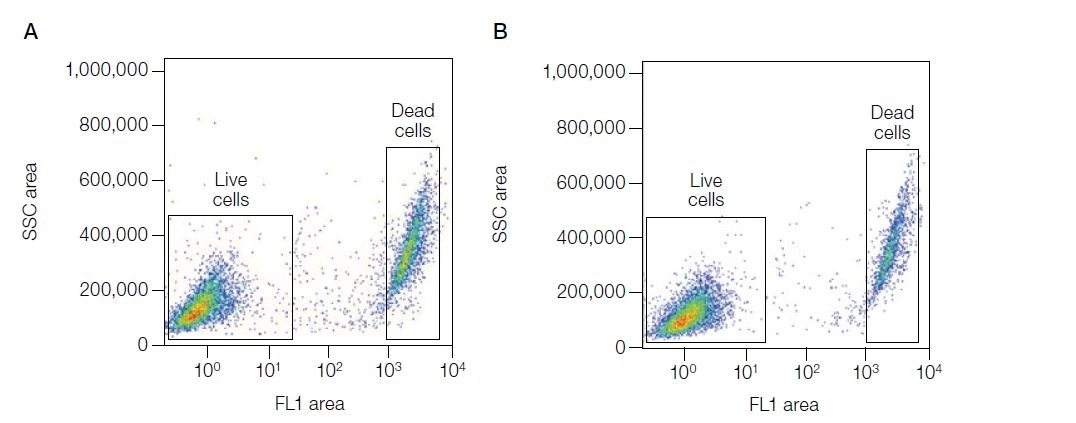
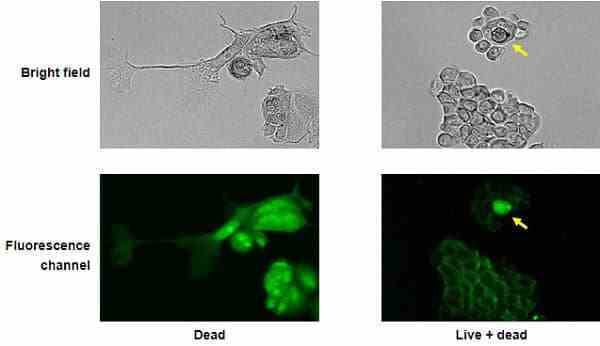
| VivaFix cell viability assays are easy-to-use, versatile solutions for assessing the viability of mammalian cells by flow cytometry and microscopy. In most flow cytometry experiments, the exclusion of dead cells within a sample is an important step to prevent erroneous interpretation of the data caused by nonspecific fluoresence signals. When performing cell imaging experiments, cell viability and live:dead ratio are parameters that are frequently evaluated to measure cell health. The proprietary dyes in the VivaFix Cell Viability Assay can easily assist researchers with distinguishing betweeen live and dead cells by covalently binding to primary amines. In live cells, the VivaFix Dyes bind to the cell surface primary amines. In dead cells, where the plasma membrane is compromised, the dyes are able to permeate the cell and also react with intracellular primary amines. As a result, a greater number of fluorophores is associated with dead cells and at least a 100-fold difference in fluorescence intensity is measured between the live and dead cells thereby allowing an easier discrimination between two populations. VivaFix Dyes are compatible with cell fixation without any significant loss of fluorescence. |
- Reagents In The Kit
- Kit includes 4 x 50 assay vials and 250 μl of DMSO, for running 200 assays
- Regulatory
- For research purposes only
- Guarantee
- Guaranteed until date of expiry. Please see product label.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | 1/500 |
- Instructions For Use
- Important: Thaw all components prior to use.
Note 1: Any buffer without sodium azide, serum or protein can be used instead of phosphate buffered saline.
Note 2: For multicolor experiments, the cells can be stained with the antibodies of your choice before or after staining with VivaFix Dye. Antibody staining conditions should be optimized for your assay.
1. Prepare a 500x stock solution by adding 50 μl of dimethyl sulfoxide (DMSO) to a VivaFix Dye vial and mix by vortexing.
2. Wash cells once with phosphate buffered saline, then resuspend cells at 2-3 x 106 cells/ml in phosphate buffered saline. Use 0.5 ml of cell suspension per assay.
3. Add 1 μl of 500x dye stock solution (from step 1) to 0.5 ml cells/assay and mix by vortexing.
4. Incubate the mixture for 30 min at room temperature (RT) or in a 37°C/5% CO2 incubator. Protect from light.
Note: The optimal dye concentration and incubation time for different cell lines should be assessed empirically.
5. Wash cells twice in phosphate buffered saline.
6. Fix cells as desired. For 3.7% formaldehyde:
-Suspend cells in 900 μl of phosphate buffered saline following step 5
-Add 100 μl of 37% of formaldehyde to the cell suspension
-Incubate for 15 min at room temperature, then wash cells twice with phosphate buffered saline
7. Resuspend cells in the appropriate flow analysis buffer.
8. Sort cells with an S3e Cell Sorter, analyze cells with a flow cytometer, view and capture an image of cells with the ZOE Fluorescent Cell Imager, or view cells with a conventional fluorescence microscope.
References for VIVAFIX™ Assay
-
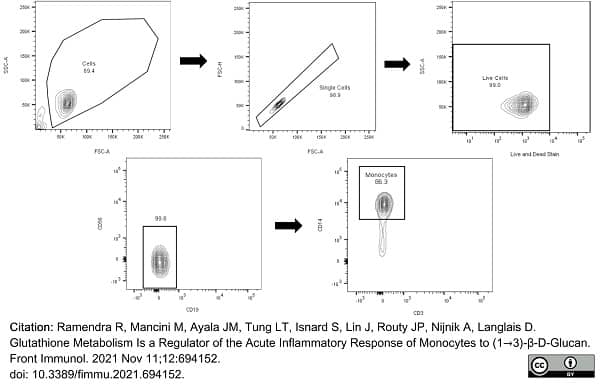
Ramendra, R. et al. (2021) Glutathione Metabolism Is a Regulator of the Acute Inflammatory Response of Monocytes to (1→3)-β-D-Glucan.
Front Immunol. 12: 694152. -
Globig, P. et al. (2021) Slow degrading Mg-based materials induce tumor cell dormancy on an osteosarcoma-fibroblast coculture model
Bioactive Materials. 30 Dec [Epub ahead of print]. -
Méndez-Solís, O. et al. (2021) Kaposi's sarcoma herpesvirus activates the hypoxia response to usurp HIF2α-dependent translation initiation for replication and oncogenesis.
Cell Rep. 37 (13): 110144. -
Manirarora, J.N. et al. (2022) Development and Characterization of New Monoclonal Antibodies Against Porcine Interleukin-17A and Interferon-Gamma
Frontiers in Immunology. 13 [Epub ahead of print]. -
Ravi, V.M. et al. (2022) T-cell dysfunction in the glioblastoma microenvironment is mediated by myeloid cells releasing interleukin-10.
Nat Commun. 13 (1): 925. -
Manirarora, J.N. et al. (2022) Development and Characterization of New Monoclonal Antibodies Against Porcine Interleukin-17A and Interferon-Gamma.
Front Immunol. 13: 786396. -
Bouch, S. et al. (2022) Therapeutic stem cell-derived alveolar-like macrophages display bactericidal effects and resolve Pseudomonas aeruginosa.-induced lung injury.
J Cell Mol Med. 26 (10): 3046-3059. -
Schlesinger, M. et al. (2022) Glucose and mannose analogs inhibit KSHV replication by blocking N-glycosylation and inducing the unfolded protein response.
J Med Virol. : e28314.
View The Latest Product References
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
Always be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up