Mycoplasma Removal Agent

Mycoplasma Removal Agent
- Product Type
- Accessory Reagent
- Specificity
- Mycoplasma Removal Agent
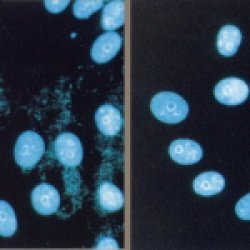
| Mycoplasma Removal Agent is a tissue culture supplement with broad anti bacterial activity intended to eliminate Mycoplasma sp. and related organisms from potentially contaminated cell cultures without inflicting damage to the cell of interest. |
- Intended Use
- MRA is very effective at removing mycoplasma from infected cell cultures. It shows strong anti-mycoplasma activity against many types of mycoplasma including Mycoplasma orale, M. arginini, M. hyorhinis and Acholeplasma laidlawii.
MRA is also suitable for use after the removal of mycoplasma, to prevent recontamination of the culture with the original mycoplasma, at preventative doses.
This product can also be used to prevent initial infection of cells in culture by mycoplasma.
MRA is non toxic, and will not interfere with the viability or function of cells in culture. It should be emphasised that MRA should not be used as a substitute for good cell culture techniques. - Approx. Protein Concentrations
- 50ug/ml
- Regulatory
- For research purposes only
- Guarantee
- Guaranteed until date of expiry. Please see product label.
This product should be stored undiluted.
This product is photosensitive and should be protected from light.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Tissue Culture |
- Instructions For Use
- MRA is very easy to use, simply requiring incubation for a week after addition to cell cultures contaminated
by mycoplasma.
Indications for Use:
1. Add MRA to cell cultures contaminated by mycoplasma at a concentration of 0.5 μg/ml and incubate for a week.
2. For media replacement or culture transfer (passage), use a medium containing MRA at this same concentration.
3. Transfer the cell cultures several times without MRA and confirm that regrowth of the contaminating mycoplasma has not occurred.
If there is a concern about the presence of mycoplasma in serum or trypsin, MRA can be added to the media at a concentration of 0.5 μg/ml to prevent contamination of the cell cultures exposed to these products.
N.B. The recommended concentration for use is 0.5 μg/ml. The MRA concentration may be raised up to 1 μg/ml only when the recommended concentration is ineffective in removing the mycoplasma.
The cytotoxicity of MRA is low and cell toxicity is rare when used at the recommended concentration. For specific function of any cell, however, it is recommended that the retention of desired cellular characteristics be confirmed after treatment.
Please follow this link to view Sample Data.
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse Monoclonal Antibody Isotyping Test Kit | MMT1 | IS | 10 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse Monoclonal Antibody Isotyping Test Kit | ||||||
| Proteus Protein A MIDI Purification Kit | PUR003 | PP | 4 Units | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Proteus Protein A MIDI Purification Kit | ||||||
| Proteus Protein A Mini Purification Kit | PUR008 | PP | 16 Units | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Proteus Protein A Mini Purification Kit | ||||||
| Proteus Protein G MIDI Purification Kit | PUR012 | PP | 4 Units | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Proteus Protein G MIDI Purification Kit | ||||||
| Proteus Protein G Mini Purification Kit | PUR016 | PP | 16 Units | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Proteus Protein G Mini Purification Kit | ||||||
| Rat Monoclonal Antibody Isotyping Test Kit | RMT1 | IS | 10 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rat Monoclonal Antibody Isotyping Test Kit | ||||||
References for Mycoplasma Removal Agent
-
Ndungu, F.M. et al. (2006) CD4 T cells from malaria-nonexposed individuals respond to the CD36-Binding Domain of Plasmodium falciparum erythrocyte membrane protein-1 via an MHC class II-TCR-independent pathway.
J Immunol. 176 (9): 5504-12. -
Molla Kazemiha, V. et al. (2009) PCR-based detection and eradication of mycoplasmal infections from various mammalian cell lines: a local experience.
Cytotechnology. 61 (3): 117-24. -
Entrican, G. et al. (2009) Growing Hybridomas
In: Walker, J.M. (eds) The Protein Protocols Handbook. Springer Protocols Handbooks. Humana Press, Totowa, NJ. pp.1887-99. -
Molla Kazemiha, V. et al. (2011) Efficiency of Plasmocin™ on various mammalian cell lines infected by mollicutes in comparison with commonly used antibiotics in cell culture: a local experience.
Cytotechnology. 63 (6): 609-20. -
Chang, M.C. et al. (2015) N-Farnesyloxy-norcantharimide and N-farnesyl-norcantharimide inhibit the progression of leukemia and increase survival days in a syngeneic mouse leukemia model.
Anticancer Drugs. 26 (5): 508-17. -
Russell, C.L. et al. (2017) Combined tissue and fluid proteomics with Tandem Mass Tags to identify low-abundance protein biomarkers of disease in peripheral body fluid: An Alzheimer's Disease case study.
Rapid Commun Mass Spectrom. 31 (2): 153-9. -
Santana-Rivera, Y. et al. (2020) Reduced expression of enolase-1 correlates with high intracellular glucose levels and increased senescence in cisplatin-resistant ovarian cancer cells.
Am J Transl Res. 12 (4): 1275-92.
Further Reading
-
Nakai, N. et al. (2000) Detection and elimination of contaminating microorganisms in transplantable tumors and cell lines.
Exp Anim. 49 (4): 309-13.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
Always be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up