Histidine Tag antibody | AD1.1.10














Mouse anti Histidine Tag:Alexa Fluor® 647
- Product Type
- Monoclonal Antibody
- Clone
- AD1.1.10
- Isotype
- IgG1
- Specificity
- Histidine Tag
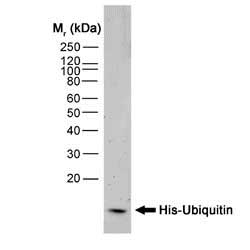
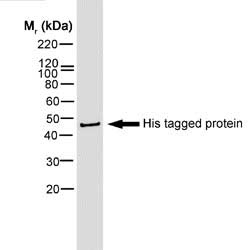
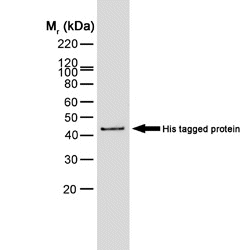
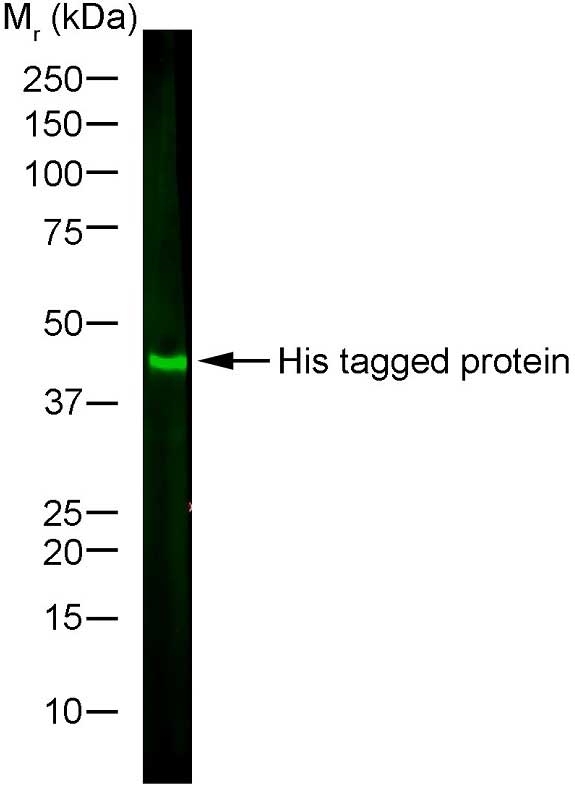
| Mouse anti Histidine tag antibody, clone AD1.1.10 recognizes proteins and peptides containing the motif H-H-H-H-H-H and is therefore of value in detecting proteins containing histidine tags. Clone AD1.1.10 has been used to detect and purify histidine-tagged proteins expressed in mammalian ( In Western blotting of bacterial extracts the antibody has been shown not to cross-react with any endogenous products, although some cross-reactivity may be seen with extracts of insect or mammalian cells. This antibody is routinely tested in Western blotting on histidine tagged recombinant proteins and reacts against all histidine-tagged proteins so far tested. |
- Target Species
- Synthetic Peptide
- Product Form
- Purified IgG conjugated to Alexa Fluor® 647 - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
1% bovine serum albumin - Immunogen
- PAX6 transcription factor linked to histidine tag.
- Approx. Protein Concentrations
- IgG concentration 0.05 mg/ml
- Fusion Partners
- Spleen cells from immunized Balb/c mice were fused with cells of the mouse NS1 myeloma cell line.
- Max Ex/Em
-
Fluorophore Excitation Max (nm) Emission Max (nm) Alexa Fluor®647 650 665 - Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
- Acknowledgements
- This product is provided under an intellectual property licence from Life Technologies Corporation. The transfer of this product is contingent on the buyer using the purchase product solely in research, excluding contract research or any fee for service research, and the buyer must not sell or otherwise transfer this product or its components for (a) diagnostic, therapeutic or prophylactic purposes; (b) testing, analysis or screening services, or information in return for compensation on a per-test basis; (c) manufacturing or quality assurance or quality control, or (d) resale, whether or not resold for use in research. For information on purchasing a license to this product for purposes other than as described above, contact Life Technologies Corporation, 5791 Van Allen Way, Carlsbad CA 92008 USA or outlicensing@thermofisher.com
His-tag is a registered trademark of EMD Biosciences.
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended. This product is photosensitive and should be protected from light.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
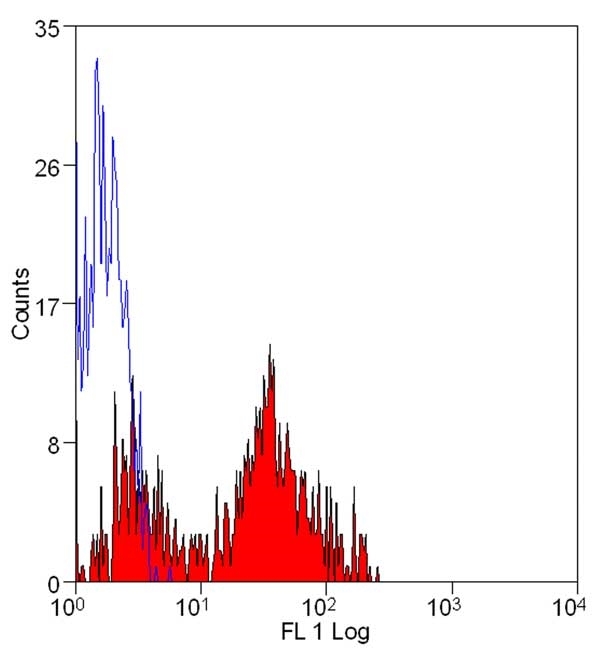
| Flow Cytometry |
References for Histidine Tag antibody
-
Els Conrath, K. et al. (2001) Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs.
J Biol Chem. 276 (10): 7346-50. -
Suen, J.L. et al. (2001) Characterization of self-T-cell response and antigenic determinant of U1A protein with bone marrow-derived dendritic cells in NZB x NZW F1 mice.
Immunol. 103: 301-309. -
Hoffmann, S.C. et al. (2007) Identification of CLEC12B, an inhibitory receptor on myeloid cells.
J Biol Chem. 282 (31): 22370-5. -
Zheng, J. et al. (2007) Serum from mice immunized in the context of Treg inhibition identifies DEK as a neuroblastoma tumor antigen.
BMC Immunol. 8: 4. -
Bahi, A. & Dreyer, J.L. (2008) Overexpression of plasminogen activators in the nucleus accumbens enhances cocaine-, amphetamine- and morphine-induced reward and behavioral sensitization.
Genes Brain Behav. 7 (2): 244-56. -
Wrighton, K.H. et al. (2009) Transforming Growth Factor {beta} Can Stimulate Smad1 Phosphorylation Independently of Bone Morphogenic Protein Receptors.
J Biol Chem. 284 (15): 9755-63. -
Diefenbacher, M. et al. (2011) The Dsl1 Tethering Complex Actively Participates in Soluble NSF (N-Ethylmaleimide-sensitive Factor) Attachment Protein Receptor (SNARE) Complex Assembly at the Endoplasmic Reticulum in Saccharomyces cerevisiae.
J Biol Chem. 286: 25027-38. -
Alvarez, M.M. et al. (2010) Specific recognition of influenza A/H1N1/2009 antibodies in human serum: a simple virus-free ELISA method.
PLoS One. 5: e10176.
View The Latest Product References
-
Bahi, A. et al. (2008) The role of tissue-type plasminogen activator system in amphetamine-induced conditional place preference extinction and reinstatement.
Neuropsychopharmacology. 33: 2726-34. -
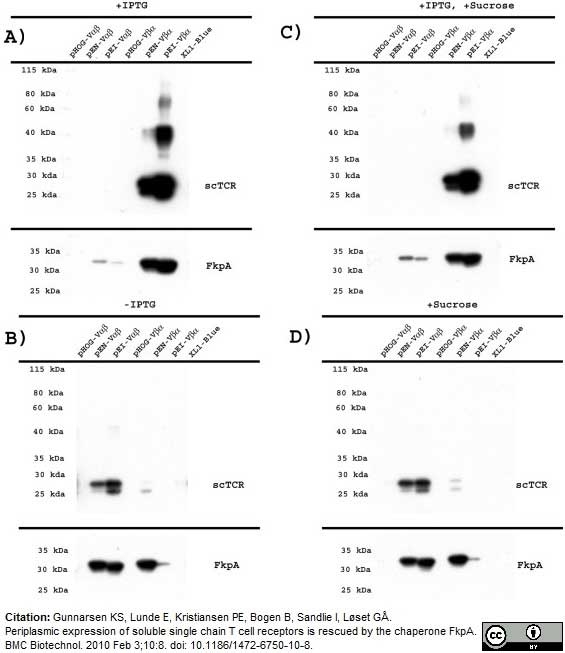
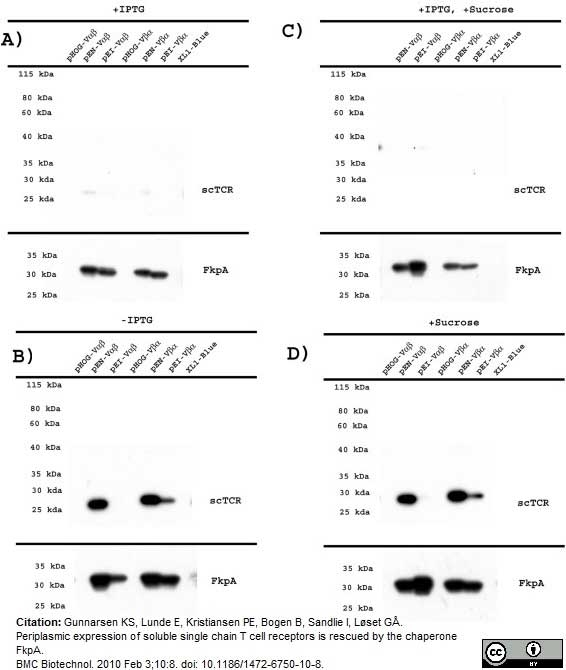
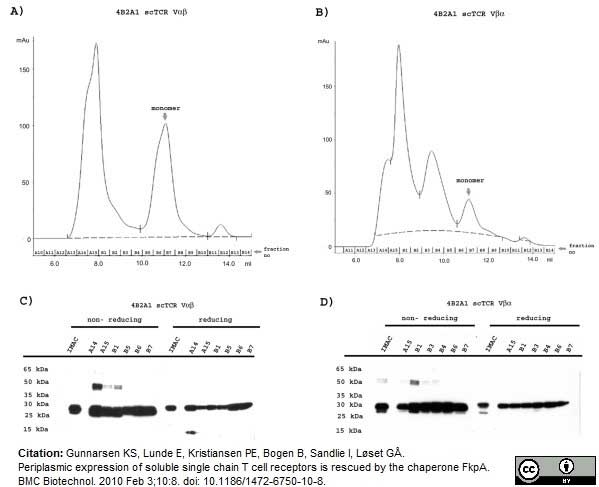
Gunnarsen, K.S. et al. (2010) Periplasmic expression of soluble single chain T cell receptors is rescued by the chaperone FkpA.
BMC Biotechnol. 10: 8. -
Hwang, H.Y. et al. (2008) Highly specific inhibition of C1q globular-head binding to human IgG: a novel approach to control and regulate the classical complement pathway using an engineered single chain antibody variable fragment.
Mol Immunol. 45: 2570-80. -
De Vooght, L. et al. (2012) Expression and extracellular release of a functional anti-trypanosome Nanobody® in Sodalis glossinidius, a bacterial symbiont of the tsetse fly.
Microb Cell Fact. 11: 23. -
Saerens, D. et al. (2004) Single domain antibodies derived from dromedary lymph node and peripheral blood lymphocytes sensing conformational variants of prostate-specific antigen.
J Biol Chem. 279 (50): 51965-72. -
Than, N.G. et al. (2014) Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia.
Placenta. 35 (11): 855-65. -
Elders RC et al. (2014) Recombinant canine IgE Fc and an IgE Fc-TRAIL fusion protein bind to neoplastic canine mast cells.
Vet Immunol Immunopathol. 159 (1-2): 29-40. -
Chin, S.E. et al. (2015) Isolation of high-affinity, neutralizing anti-idiotype antibodies by phage and ribosome display for application in immunogenicity and pharmacokinetic analyses.
J Immunol Methods. 416: 49-58. -
Peyrassol, X. et al. (2016) Development by Genetic Immunization of Monovalent Antibodies (Nanobodies) Behaving as Antagonists of the Human ChemR23 Receptor.
J Immunol. 196 (6): 2893-901. -
Kim H & Loparo JJ (2016) Multistep assembly of DNA condensation clusters by SMC.
Nat Commun. 7: 10200. -
Borg M et al. (2014) A novel interaction between Rab7b and actomyosin reveals a dual role in intracellular transport and cell migration.
J Cell Sci. 127 (Pt 22): 4927-39. -
De Meyer, T. et al. (2015) Comparison of VHH-Fc antibody production in Arabidopsis thaliana, Nicotiana benthamiana and Pichia pastoris.
Plant Biotechnol J. 13 (7): 938-47. -
Siddiqui AA et al. (2015) Humoral immune responses to a recombinant Plasmodium vivax tryptophan-rich antigen among Plasmodium vivax-infected patients and its localization in the parasite.
Appl Biochem Biotechnol. 175 (4): 2166-77. -
Warnecke, A. et al. (2017) Nitration of MOG diminishes its encephalitogenicity depending on MHC haplotype.
J Neuroimmunol. 303: 1-12. -
Bertucci, A. et al. (2011) A new coral carbonic anhydrase in Stylophora pistillata.
Mar Biotechnol (NY). 13 (5): 992-1002. -
Boujon, C.L. et al. (2017) Development and validation of an immunohistochemistry procedure for the detection of a neurotropic bovine astrovirus.
J Virol Methods. 239: 26-33. -
Cartwright, S.P. et al. (2017) Rapid expression and purification of the hepatitis delta virus antigen using the methylotropic yeast Pichia pastoris.
BMC Res Notes. 10 (1): 340. -
Thanongsaksrikul, J. et al. (2018) Identification and production of mouse scFv to specific epitope of enterovirus-71 virion protein-2 (VP2).
Arch Virol. 163 (5): 1141-1152. -
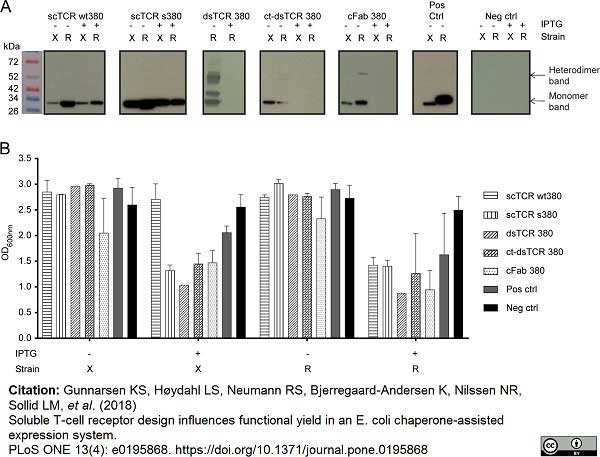
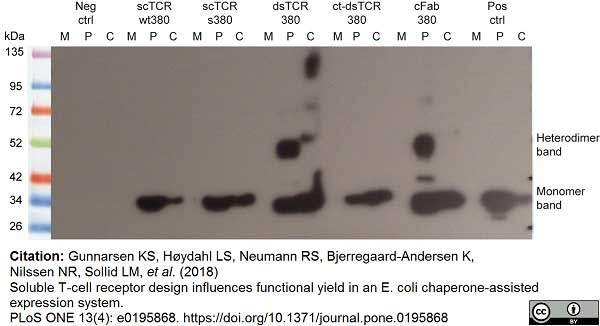
Gunnarsen, K.S. et al. (2018) Soluble T-cell receptor design influences functional yield in an E. coli chaperone-assisted expression system.
PLoS One. 13 (4): e0195868. -
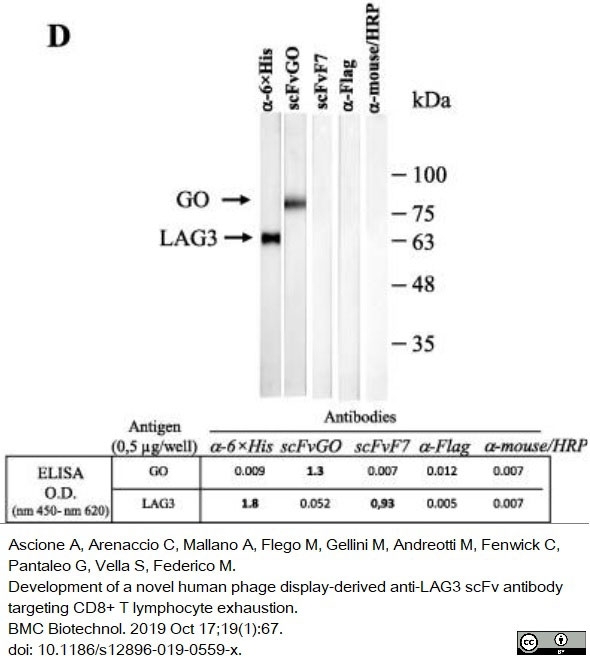
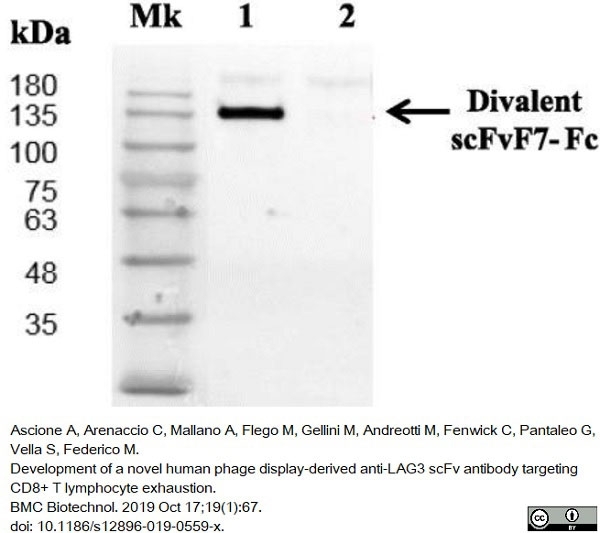
Ascione, A. et al. (2019) Development of a novel human phage display-derived anti-LAG3 scFv antibody targeting CD8+ T lymphocyte exhaustion.
BMC Biotechnol. 19 (1): 67. -
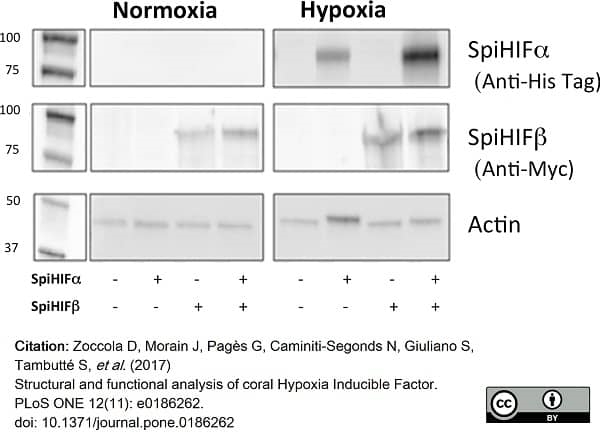
Zoccola, D. et al. (2017) Structural and functional analysis of coral Hypoxia Inducible Factor.
PLoS One. 12 (11): e0186262. -
Kimura, K. et al. (2021) Overexpression of human BAG3P209L in mice causes restrictive cardiomyopathy.
Nat Commun. 12 (1): 3575. -
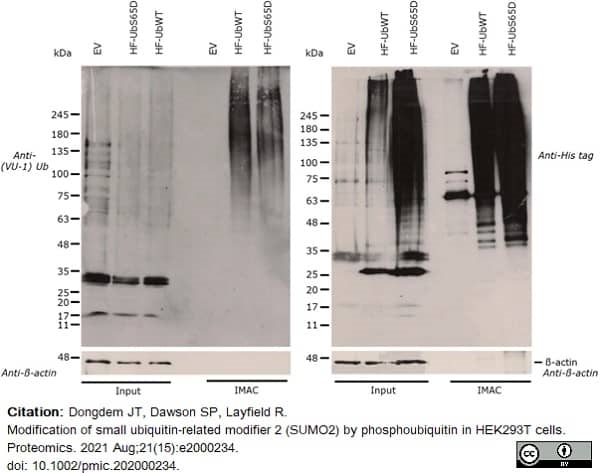
Dongdem, J.T. et al. (2021) Modification of small ubiquitin-related modifier 2 (SUMO2) by phosphoubiquitin in HEK293T cells.
Proteomics. 21 (15): e2000234. -
Chuang, H.C. et al. (2021) Effect of cell-permeable grouper Manganese Superoxide Dismutase on environmental stress in fish.
Protein Expr Purif. 187: 105951. -
Cheng, C.M. et al. (2021) Heterologous expression of bacterial CotA-laccase, characterization and its application for biodegradation of malachite green.
Bioresour Technol. 340: 125708. -
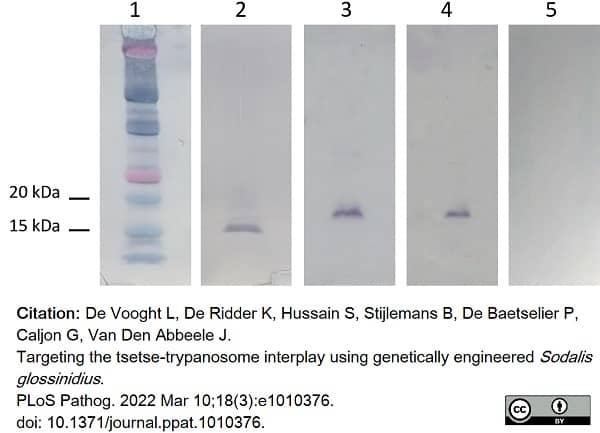
De Vooght, L. et al. (2022) Targeting the tsetse-trypanosome interplay using genetically engineered Sodalis glossinidius..
PLoS Pathog. 18 (3): e1010376. -
Minami, S.A. et al. (2022) Production of novel SARS-CoV-2 Spike truncations in Chinese hamster ovary cells leads to high expression and binding to antibodies.
Biotechnol J. 17 (9): e2100678. -
Chen, Y.J. et al. (2023) A non-genetic engineering platform for rapidly generating and expanding cancer-specific armed T cells.
J Biomed Sci. 30 (1): 35. -
Boudkkazi, S. et al. (2023) A Noelin-organized extracellular network of proteins required for constitutive and context-dependent anchoring of AMPA-receptors.
Neuron. 111 (16): 2544-56.e9. -
Nguyen, H.M. et al. (2023) Heterologous expression and characterization of a MoAA16 polysaccharide monooxygenase from the rice blast fungus Magnaporthe oryzae
Electronic Journal of Biotechnology. 66: 1-16. -
Rossey, I. et al. (2021) A vulnerable, membrane-proximal site in human respiratory syncytial virus F revealed by a prefusion-specific single-domain antibody.
J Virol. 95 (11): e02279-20. -
Khosravi, M. et al. (2016) Canine Distemper Virus Fusion Activation: Critical Role of Residue E123 of CD150/SLAM.
J Virol. 90 (3): 1622-37. -
Tamaki, Y. et al. (2024) Shiga toxin type 2 B subunit protects mice against toxin challenge when leashed and bundled by a stable pentameric coiled-coil molecule.
Vaccine. Feb 15 S0264-410X(24)00129-4. [Epub ahead of print]. -
Kimura, T. et al. (2024) Quantification of lipoprotein lipase in mouse plasma with a sandwich enzyme-linked immunosorbent assay.
J Lipid Res. 65 (4): 100532.
- RRID
- AB_906039
MCA1396A647
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Synthetic Peptide ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up