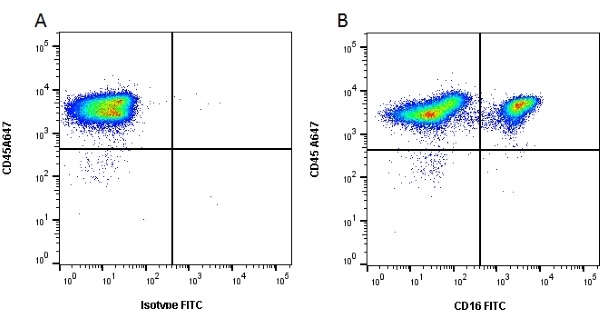
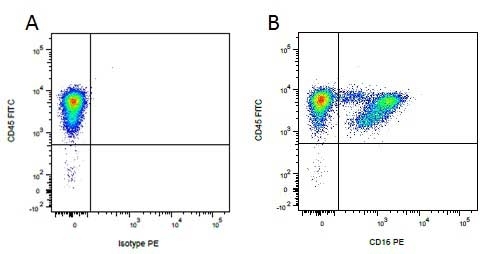
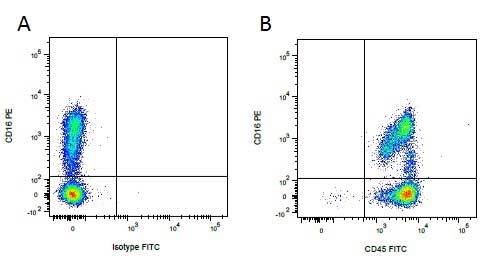
CD16 antibody | G7






Mouse anti Pig CD16:FITC
- Product Type
- Monoclonal Antibody
- Clone
- G7
- Isotype
- IgG1
- Specificity
- CD16
| Mouse anti Pig CD16, clone G7 recognizes porcine CD16 also known as Fc-gamma RIII or the low affinity IgG (Fc) receptor III. Clone G7 was clustered as CD16 at the Second International Workshop to Define Swine Cluster of Differentiation (CD) Antigens (Saalmuller et al. 1998). Mouse anti pig CD16 immunoprecipitates a protein of ~40 kDa from porcine neutrophils and NK cells (Wierda et al. 1993). Subsequent cloning and characterization of the G7 molecule indicated that G7 was the porcine homologue of Human CD16 (Halloran et al. 1994). |
- Target Species
- Pig
- Product Form
- Purified IgG conjugated to Fluorescein Isothiocyanate Isomer 1 (FITC) - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
1% bovine serum albumin - Immunogen
- Porcine peripheral blood leucocytes
- Approx. Protein Concentrations
- IgG concentration 0.1 mg/ml
- Fusion Partners
- Spleen cells from immunized Balb/c mice were fused with cells of the mouse P3-X63-Ag8.653 myeloma cell line
- Max Ex/Em
-
Fluorophore Excitation Max (nm) Emission Max (nm) FITC 490 525 - Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended. This product is photosensitive and should be protected from light.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | Neat |
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells in 100μl
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG1 Negative Control:FITC | MCA928F | F | 100 Tests |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Mouse IgG1 Negative Control:FITC | ||||||
Source Reference
-
Dato, M.E. et al. (1992) A triggering structure recognized by G7 monoclonal antibody on porcine lymphocytes and granulocytes.
Cell Immunol. 140 (2): 468-77.
References for CD16 antibody
-
Wierda, W.G. et al. (1993) Two distinct porcine natural killer lytic trigger molecules as PNK-E/G7 molecular complex.
Cell Immunol. 146 (2): 270-83. -
Halloran, P.J. et al. (1994) Biochemical characterization of the porcine Fc gamma RIII alpha homologue G7.
Cell Immunol. 158 (2): 400-13. -
Sánchez, C. et al. (1999) The porcine 2A10 antigen is homologous to human CD163 and related to macrophage differentiation.
J Immunol. 162 (9): 5230-7. -
Terzic, S. et al. (2002) Immunophenotyping of leukocyte subsets in peripheral blood and palatine tonsils of prefattening pigs.
Vet Res Commun. 26: 273 - 83. -
Vincent, I.E. et al. (2003) Dendritic cells harbor infectious porcine circovirus type 2 in the absence of apparent cell modulation or replication of the virus.
J Virol. 77: 13288 - 300. -
Summerfield, A. et al. (2003) Porcine peripheral blood dendritic cells and natural interferon-producing cells.
Immunology 110: 440-9. -
Inman, C.F. et al. (2010) Rearing environment affects development of the immune system in neonates.
Clin Exp Immunol. 160 (3): 431-9. -
Inman, C.F. et al. (2010) Dendritic cells interact with CD4 T cells in intestinal mucosa.
J Leukoc Biol. 88 (3): 571-8.
View The Latest Product References
-
Devriendt, B. et al. (2010) Targeting of Escherichia coli F4 fimbriae to Fcgamma receptors enhances the maturation of porcine dendritic cells.
Vet Immunol Immunopathol. 135 (3-4): 188-98. -
Gimeno, M. et al. (2011) Cytokine profiles and phenotype regulation of antigen presenting cells by genotype-I porcine reproductive and respiratory syndrome virus isolates.
Vet Res. 42: 9. -
Mussá, T. et al. (2011) Interaction of porcine conventional dendritic cells with swine influenza virus.
Virology 420: 125-34. -
Lecours, M.P. et al. (2011) Characterization of porcine dendritic cell response to Streptococcus suis.
Vet Res. 42: 72. -
Mair, K.H. et al. (2012) NKp46 expression discriminates porcine NK cells with different functional properties.
Eur J Immunol. 42: 1261-71. -
Hester, S.N. et al. (2012) Intestinal and systemic immune development and response to vaccination are unaffected by dietary (1,3/1,6)-β-D-glucan supplementation in neonatal piglets.
Clin Vaccine Immunol. 19 (9): 1499-508. -
Thierry, A. et al. (2012) Identification of invariant natural killer T cells in porcine peripheral blood.
Vet Immunol Immunopathol. 149 (3-4): 272-9. -
Inman, C.F. et al. (2012) Neonatal colonisation expands a specific intestinal antigen-presenting cell subset prior to CD4 T-cell expansion, without altering T-cell repertoire.
PLoS One 7: e33707. -
Masure, D. et al. (2013) A Role for Eosinophils in the Intestinal Immunity against Infective Ascaris suum Larvae.
PLoS Negl Trop Dis. 7: e2138. -
Mair, K.H. et al. (2013) Porcine CD8αdim/-NKp46high NK cells are in a highly activated state.
Vet Res. 44: 13. -
Kyrova, K. et al. (2014) The response of porcine monocyte derived macrophages and dendritic cells to Salmonella typhimurium and lipopolysaccharide.
BMC Vet Res. 10: 244. -
Waide, E.H. et al. (2015) Not All SCID Pigs Are Created Equally: Two Independent Mutations in the Artemis Gene Cause SCID in Pigs.
J Immunol. 195 (7): 3171-9. -
Auray, G. et al. (2016) Characterization and Transcriptomic Analysis of Porcine Blood Conventional and Plasmacytoid Dendritic Cells Reveals Striking Species-Specific Differences.
J Immunol. 197 (12): 4791-806. -
Suzuki, S. et al. (2016) Generation and characterization of RAG2 knockout pigs as animal model for severe combined immunodeficiency.
Vet Immunol Immunopathol. 178: 37-49. -
LeLuduec, J.B. et al. (2016) Intradermal vaccination with un-adjuvanted sub-unit vaccines triggers skin innate immunity and confers protective respiratory immunity in domestic swine.
Vaccine. 34 (7): 914-22. -
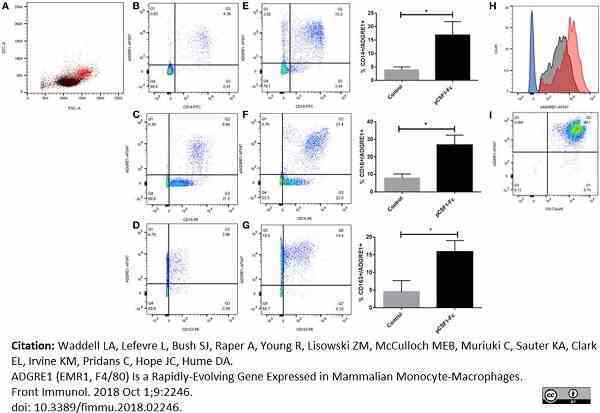
Waddell, L.A. et al. (2018) ADGRE1 (EMR1, F4/80) Is a Rapidly-Evolving Gene Expressed in Mammalian Monocyte-Macrophages.
Front Immunol. 9: 2246. -
Fernández-Caballero, T. et al. (2018) Phenotypic and functional characterization of porcine bone marrow monocyte subsets.
Dev Comp Immunol. 81: 95-104. -
Loss, H. et al. (2018) Effects of a pathogenic ETEC strain and a probiotic Enterococcus faecium strain on the inflammasome response in porcine dendritic cells.
Vet Immunol Immunopathol. 203: 78-87. -
Skovdal, S.M. et al. (2019) Inhaled nebulized glatiramer acetate against Gram-negative bacteria is not associated with adverse pulmonary reactions in healthy, young adult female pigs.
PLoS One. 14 (10): e0223647. -
Ferret-Bernard, S. et al. (2020) Maternal Supplementation of Food Ingredient (Prebiotic) or Food Contaminant (Mycotoxin) Influences Mucosal Immune System in Piglets.
Nutrients. 12 (7): 2115. -
Boettcher, A.N. et al. (2020) CD3ε(+) Cells in Pigs With Severe Combined Immunodeficiency Due to Defects in ARTEMIS.
Front Immunol. 11: 510. -
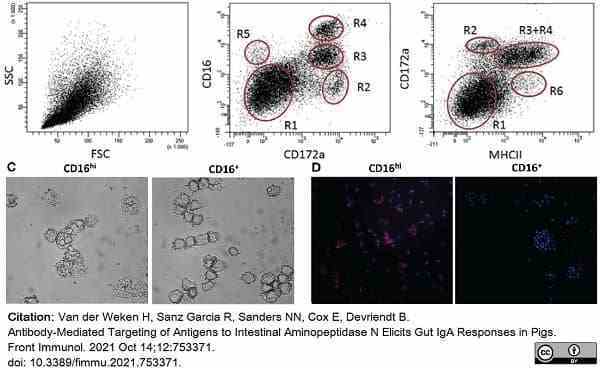
Van der Weken, H. et al. (2021) Antibody-Mediated Targeting of Antigens to Intestinal Aminopeptidase N Elicits Gut IgA Responses in Pigs.
Front Immunol. 12: 753371. -
Teuben, M. et al. (2021) Instant intra-operative neutropenia despite the emergence of banded (CD16dim/CD62Lbright) neutrophils in peripheral blood - An observational study during extensive trauma-surgery in pigs.
Injury. 52 (3): 426-33. -
Teuben, M.P.J. et al. (2022) Standardized porcine unilateral femoral nailing is associated with changes in PMN activation status, rather than aberrant systemic PMN prevalence.
Eur J Trauma Emerg Surg. 48 (3): 1601-11. -
Zhao, H. et al. (2022) Development of RAG2 -/- IL2Rγ -/Y immune deficient FAH-knockout miniature pig.
Front Immunol. 13: 950194. -
Štěpánová, H. et al. (2022) Characterization of Porcine Monocyte-Derived Macrophages Cultured in Serum-Reduced Medium.
Biology (Basel). 11(10):1457. -
Monguió-Tortajada, M. et al. (2022) Acellular cardiac scaffolds enriched with MSC-derived extracellular vesicles limit ventricular remodelling and exert local and systemic immunomodulation in a myocardial infarction porcine model.
Theranostics. 12 (10): 4656-70. -
Haach, V. et al. (2023) A polyvalent virosomal influenza vaccine induces broad cellular and humoral immunity in pigs.
Virol J. 20 (1): 181. -
Álvarez, B. et al. (2023) Porcine Macrophage Markers and Populations: An Update.
Cells. 12 (16): 2103.
Further Reading
-
Piriou-Guzylack, L. (2008) Membrane markers of the immune cells in swine: an update.
Vet Res. 39: 54. -
Gerner W et al. (2015) Phenotypic and functional differentiation of porcine αβ T cells: current knowledge and available tools.
Mol Immunol. 66 (1): 3-13.
- Synonyms
- FcRIII
- RRID
- AB_2104030
- UniProt
- Q28942
- Entrez Gene
- FCGR3B
- GO Terms
- GO:0005886 plasma membrane
- GO:0016021 integral to membrane
- GO:0004872 receptor activity
- GO:0019864 IgG binding
MCA1971F
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Pig ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up