Cytochrome P450 Aromatase antibody | H4












Mouse anti Human Cytochrome P450 Aromatase
- Product Type
- Monoclonal Antibody
- Clone
- H4
- Isotype
- IgG2a
- Specificity
- Cytochrome P450 Aromatase
| Mouse anti Human Cytochrome P450 aromatase antibody, clone H4 recognizes a conserved epitope within cytochrome P450 aromatase (P450 arom). P450 arom plays an important role in estrogen biosynthesis and is highly conserved amongst mammals. P450 arom is highly expressed in placental tissue. For tissues where there may be low expression of P450 arom, the use of microsomal extracts may improve the staining for Western blots using Mouse anti Human Cytochrome P450 aromatase antibody, clone H4 (Turner et al. 2002). |
- Target Species
- Human
- Species Cross-Reactivity
-
Target Species Cross Reactivity Rat Marmoset Chicken Mouse Pig Baboon Bovine Horse Great fruit eating bat Rabbit Sheep Collared peccary Goat Giraffe Minke whale Bryde's whale Sei whale Impala - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Concentrated tissue culture supernatant - liquid
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Immunogen
- Synthetic peptide corresponding to amino acids 376 - 390 of human aromatase.
- Fusion Partners
- Spleen cells from immunized Balb/c were fused with with cells of the mouse SP20 myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Immunofluorescence | |||
| Immunohistology - Paraffin | |||
| Western Blotting | 1/250 |
- Histology Positive Control Tissue
- Human placenta
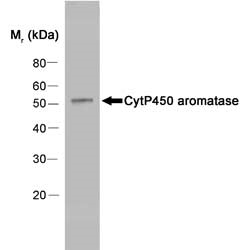
- Western Blotting
- Mouse anti Human cytochrome p450 aromatase antibody, clone H4 detects a band of approximately 55 kDa in human placental extracts.
References for Cytochrome P450 Aromatase antibody
-
(2011) Human Protein Atlas: CAB000355
Human Protein Atlas: CAB000355 -
Hanoux, V. et al. (2003) Differential regulation of two 3' end variants of P450 aromatase transcripts and of a new truncated aromatase protein in rabbit preovulatory granulosa cells.
Endocrinology. 144: 4790-8. -
Fazleabas, A.T. et al. (2003) Steroid receptor and aromatase expression in baboon endometriotic lesions.
Fertil Steril. 80: 820-7. -
Carpino, A. et al. (2004) Aromatase immunolocalization in human ductuli efferentes and proximal ductus epididymis.
J Anat. 204: 217-20. -
Schmidt, M. et al. (2005) Androgen conversion in osteoarthritis and rheumatoid arthritis synoviocytes--androstenedione and testosterone inhibit estrogen formation and favor production of more potent 5alpha-reduced androgens.
Arthritis Res Ther. 7: R938-48. -
Hu, Y. et al. (2005) Modulation of aromatase expression by BRCA1: a possible link to tissue-specific tumor suppression.
Oncogene. 24: 8343-8. -
Rago, V. et al. (2005) Cytochrome P450 aromatase expression in human seminoma.
Reprod Biol Endocrinol. 3: 72. -
Pakarainen, T. et al. (2005) Knockout of luteinizing hormone receptor abolishes the effects of follicle-stimulating hormone on preovulatory maturation and ovulation of mouse graafian follicles.
Mol Endocrinol. 19: 2591-602.
View The Latest Product References
-
Pannetier, M. et al. (2006) FOXL2 activates P450 aromatase gene transcription: towards a better characterization of the early steps of mammalian ovarian development.
J Mol Endocrinol. 36: 399-413. -
Mayor, P. et al. (2006) Ovarian features of the wild collared peccary (Tayassu tajacu) from the northeastern Peruvian Amazon.
Gen Comp Endocrinol. 147: 268-75. -
Tosca, L. et al. (2006) Metformin-induced stimulation of adenosine 5' monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells.
Biol Reprod. 75: 342-51. -
Mann, G.E. et al. (2007) Identification of elevated concentrations of estradiol in bovine uterine endometrium.
Domest Anim Endocrinol. 33: 437-41. -
Lu, Y. et al. (2007) Ubiquitination and proteasome-mediated degradation of BRCA1 and BARD1 during steroidogenesis in human ovarian granulosa cells.
Mol Endocrinol. 21 (3): 651-63. -
Rago, V. et al. (2007) Cytochrome P450arom, androgen and estrogen receptors in pig sperm
Reprod Biol Endocrinol. 5: 23. -
Rajhans, R. et al. (2008) Modulation of in situ estrogen synthesis by proline-, glutamic acid-, and leucine-rich protein-1: potential estrogen receptor autocrine signaling loop in breast cancer cells.
Mol Endocrinol. 22: 649-64. -
Boorjian, S.A. et al. (2008) Hormone receptor expression in renal angiomyolipoma: clinicopathologic correlation.
Urology. 72: 927-32. -
Ghosh, S. et al. (2008) A Role of CREB in BRCA1 Constitutive Promoter Activity and Aromatase Basal Expression.
Int J Biomed Sci. 4 (4): 260-5. -
Inaoka, Y. et al. (2008) Regulation of P450 oxidoreductase by gonadotropins in rat ovary and its effect on estrogen production.
Reprod Biol Endocrinol. 6: 62. -
Moreau, F. et al. (2009) Aromatase expression in the normal human adult adrenal and in adrenocortical tumors: biochemical, immunohistochemical, and molecular studies.
Eur J Endocrinol. 160: 93-9. -
Barone, I. et al. (2009) Expression of the K303R estrogen receptor-alpha breast cancer mutation induces resistance to an aromatase inhibitor via addiction to the PI3K/Akt kinase pathway.
Cancer Res. 69: 4724-32. -
Rice, S. et al. (2009) Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway.
Endocrinology. 150: 4794-801. -
Wang, H. et al. (2009) The alternative noncoding exons 1 of aromatase (Cyp19) gene modulate gene expression in a posttranscriptional manner.
Endocrinology. 150 (7): 3301-7. -
Sirianni, R. et al. (2009) Inhibition of cyclooxygenase-2 down-regulates aromatase activity and decreases proliferation of Leydig tumor cells.
J Biol Chem. 284: 28905-16. -
Catalano, S. et al. (2009) Rapid estradiol/ERalpha signaling enhances aromatase enzymatic activity in breast cancer cells.
Mol Endocrinol. 23: 1634-45. -
Mlodawska, W. and Slomczynska, M. (2010) Immunohistochemical localization of aromatase during the development and atresia of ovarian follicles in prepubertal horses.
Theriogenology. 74: 1707-12. -
Catalano, S. et al. (2010) Farnesoid X receptor, through the binding with steroidogenic factor 1-responsive element, inhibits aromatase expression in tumor Leydig cells.
J Biol Chem. 285: 5581-93. -
Zhao, D. et al. (2010) Somatic sex identity is cell autonomous in the chicken.
Nature. 464: 237-42. -
Jeong, J.H. et al. (2010) The gene for aromatase, a rate-limiting enzyme for local estrogen biosynthesis, is a downstream target gene of Runx2 in skeletal tissues.
Mol Cell Biol. 30: 2365-75. -
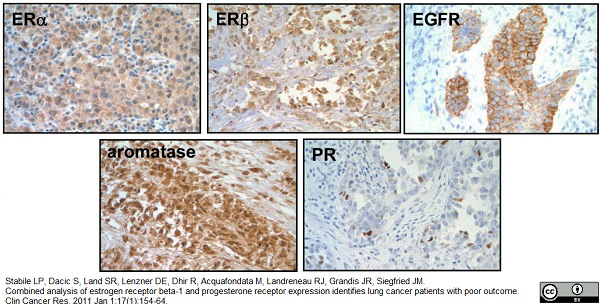
Stabile, L.P. et al. (2011) Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome.
Clin Cancer Res. 17: 154-64. -
Lu, Y. et al. (2011) BRCA1/BARD1 complex interacts with steroidogenic factor 1--A potential mechanism for regulation of aromatase expression by BRCA1.
J Steroid Biochem Mol Biol. 123: 71-8. -
Wu, Y.G. et al. (2011) Testosterone, not 5{alpha}-Dihydrotestosterone, Stimulates LRH-1 Leading to FSH-Independent Expression of Cyp19 and P450scc in Granulosa Cells.
Mol Endocrinol. 25: 656-68. -
Gallet, C. et al. (2011) The infusion of glucose in ewes during the luteal phase increases the number of follicles but reduces oestradiol production and some correlates of metabolic function in the large follicles.
Anim Reprod Sci.127: 154-63. -
Grzesiak, M. et al. (2012) Elevated level of 17β-estradiol is associated with overexpression of FSHR, CYP19A1, and CTNNB1 genes in porcine ovarian follicles after prenatal and neonatal flutamide exposure.
Theriogenology. 78: 2050-60. -
Oki, Y. et al. (2012) Dedifferentiated follicular granulosa cells derived from pig ovary can transdifferentiate into osteoblasts.
Biochem J. 447: 239-48. -
Stabile, L.P. et al. (2012) Prevention of Tobacco Carcinogen-Induced Lung Cancer in Female Mice Using Anti-Estrogens.
Carcinogenesis. 33: 2181-9. -
Oliveira, R.L. et al. (2012) Seasonal variation in estrogen receptor ERα, but not ERβ, androgen receptor and aromatase, in the efferent ductules and epididymis of the big fruit-eating bat Artibeus lituratus.
Gen Comp Endocrinol. 179: 1-13. -
Campbell, B.K. et al. (2012) The role of anti-Müllerian hormone (AMH) during follicle development in a monovulatory species (sheep).
Endocrinology. 153: 4533-43. -
Lanzino, M. et al. (2013) DAX-1, as an androgen-target gene, inhibits aromatase expression: a novel mechanism blocking estrogen-dependent breast cancer cell proliferation.
Cell Death Dis. 4: e724. -
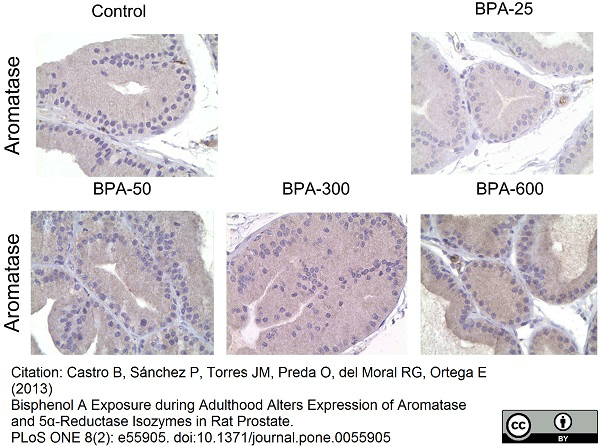
Castro, B. et al. (2013) Bisphenol A exposure during adulthood alters expression of aromatase and 5α-reductase isozymes in rat prostate.
PLoS One. 8: e55905. -
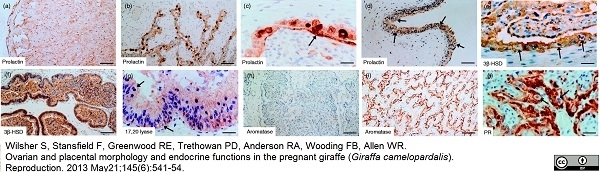
Wilsher, S. et al. (2013) Ovarian and placental morphology and endocrine functions in the pregnant giraffe (Giraffa camelopardalis).
Reproduction. 145: 541-54. -
Kim, S.O. et al. (2014) Prostaglandin E2 (EP) receptors mediate PGE2-specific events in ovulation and luteinization within primate ovarian follicles.
Endocrinology. 155 (4): 1466-75. -
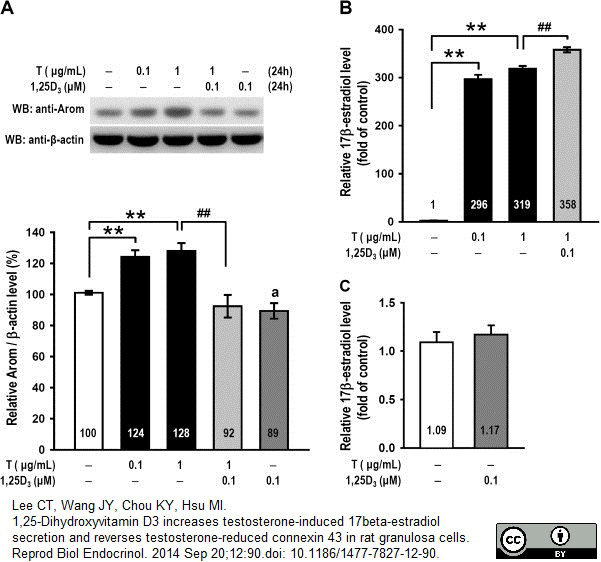
Lee, C.T. et al. (2014) 1,25-dihydroxyvitamin D3 increases testosterone-induced 17beta-estradiol secretion and reverses testosterone-reduced connexin 43 in rat granulosa cells.
Reprod Biol Endocrinol. 12: 90. -
Jiang, C. et al. (2015) Hypoglycosylated hFSH Has Greater Bioactivity Than Fully Glycosylated Recombinant hFSH in Human Granulosa Cells.
J Clin Endocrinol Metab. 100 (6): E852-60. -
Scaramuzzi, R.J. et al. (2015) The effects of intravenous, glucose versus saline on ovarian follicles and their levels of some mediators of insulin signalling.
Reprod Biol Endocrinol. 13 (1): 6. -
Garcia-Morales, C. et al. (2015) Cell-autonomous sex differences in gene expression in chicken bone marrow-derived macrophages.
J Immunol. 194 (5): 2338-44. -
Kitayama C et al. (2015) Structure and functions of the placenta in common minke (Balaenoptera acutorostrata), Bryde's (B. brydei) and sei (B. borealis) whales.
J Reprod Dev. 61 (5): 415-21. -
Kobayashi, H. et al. (2016) Gastric 17β-estradiol in portal vein and liver Esr1 make a circadian rhythm in systemic circulation in male rats.
Endocrine. 53 (2): 565-73. -
Panza S et al. (2016) Glucocorticoid Receptor as a Potential Target to Decrease Aromatase Expression and Inhibit Leydig Tumor Growth.
Am J Pathol. 186 (5): 1328-39. -
Kato, N. et al. (2016) Expression of P450 Aromatase in Granulosa Cell Tumors and Sertoli-Stromal Cell Tumors of the Ovary: Which Cells Are Responsible for Estrogenesis?
Int J Gynecol Pathol. 35 (1): 41-7. -
Roche, J. et al. (2016) Apelin (APLN) and Apelin Receptor (APLNR) in Human Ovary: Expression, Signaling, and Regulation of Steroidogenesis in Primary Human Luteinized Granulosa Cells.
Biol Reprod. 95 (5): 104. -
Tabęcka-Łonczyńska, A. et al. (2016) Retrograde and destination transfer of sex steroid hormones in the spermatic cord vessels of the mature boar (Sus scrofa) in short-daylight and long-daylight periods, as well as vernal and autumnal equinox.
Anim Reprod Sci. 164: 1-8. -
Baravalle, R. et al. (2017) Impact of R264C and R264H polymorphisms in human aromatase function.
J Steroid Biochem Mol Biol. 167: 23-32. -
Nguyen, D.P. et al. (2017) Association of Aromatase With Bladder Cancer Stage and Long-Term Survival: New Insights Into the Hormonal Paradigm in Bladder Cancer.
Clin Genitourin Cancer. 15 (2): 256-262.e1. -
Bai, L. et al. (2020) BMP2 increases the production of BDNF through the upregulation of proBDNF and furin expression in human granulosa-lutein cells.
FASEB J. 34 (12): 16129-43. -
Fang, Y. et al. (2020) Virus analog decreases estradiol secretion in FSH-treated human ovarian granulosa cells.
Gynecol Endocrinol. 36 (4): 346-50. -
Wilsher, S. et al. (2020) Placentation and hormonal maintenance of pregnancy in the impala (Aepyceros melampus.).
Placenta. 95: 91-105. -
Grzesiak, M. et al. (2021) Effect of dietary supplementation with nettle or fenugreek on folliculogenesis and steroidogenesis in the rabbit ovary - An in vivo. study.
Theriogenology. 173: 1-11. -
Kobayashi, H. et al. (2021) Estrogen synthesis in the stomach of Sprague-Dawley rats: comparison to Wistar rats.
Exp Anim. 70 (1): 63-72. -
Shiraishi, K. et al. (2021) Testicular Testosterone and Estradiol Concentrations and Aromatase Expression in Men with Nonobstructive Azoospermia.
J Clin Endocrinol Metab. 106 (4): e1803-e1815. -
Kobayashi, H. et al. (2022) Age-related alterations of gastric mucosa and estrogen synthesis in rat parietal cells.
Histochem Cell Biol. 157 (2): 195-204. -
Sánchez, P. et al. (2022) Impact of chronic exposure of rats to bisphenol A from perinatal period to adulthood on intraprostatic levels of 5α-reductase isozymes, aromatase, and genes implicated in prostate cancer development.
Environ Res. 212 (Pt A): 113142. -
Serra, L. et al. (2023) In vitro exposure to triazoles used as fungicides impairs human granulosa cells steroidogenesis.
Environ Toxicol Pharmacol. 104: 104295. -
Oczkowski, M. et al. (2023) Does Nanosilver Exposure Modulate Steroid Metabolism in the Testes?—A Possible Role of Redox Balance Disruption
Biomedicines. 12 (1): 73. -
Machaliński, B. et al. (2020) Transcriptome Profile of Human Fibroblasts in an Ex Vivo Culture.
Int J Med Sci. 17 (1): 125-36. -
Lewchalermwong, D. et al. (2020) Investigation into the variation in follicular and endocrine responses of prepubertal gilts treated with exogenous gonadotropins.
Anim Reprod Sci. 223: 106622. -
Wilsher, S. et al. (2019) Placentation in the Blue Wildebeest (Connochaetes taurinus).
Placenta. 82: 46-56. -
Jones, C.J.P. et al. (2019) A preliminary study of the heterogeneity in endometrial morphology and glycosylation in the uterine horns of the non-pregnant impala (Aepyceros melampus).
Anim Reprod Sci. 204: 66-75. -
Fang, L. et al. (2019) Human chorionic gonadotropin-induced amphiregulin stimulates aromatase expression in human granulosa-lutein cells: a mechanism for estradiol production in the luteal phase.
Hum Reprod. 34 (10): 2018-26. -
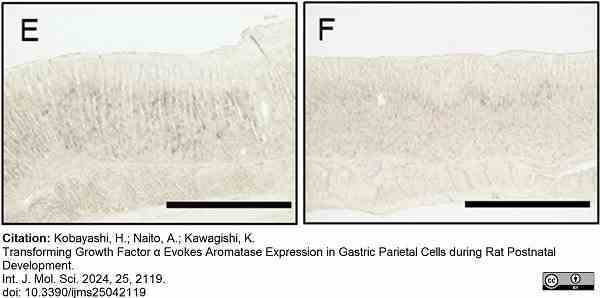
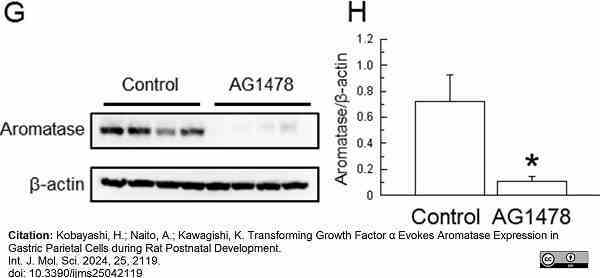
Kobayashi, H. et al. (2024) Transforming Growth Factor α Evokes Aromatase Expression in Gastric Parietal Cells during Rat Postnatal Development
International Journal of Molecular Sciences. 25 (4): 2119. -
Lund, M. et al. (2023) Luteinizing hormone receptor promotes angiogenesis in ovarian endothelial cells of Macaca fascicularis and Homo sapiens†.
Biol Reprod. 108 (2): 258-68.
- RRID
- AB_566942
- UniProt
- P11511
- Entrez Gene
- CYP19A1
- GO Terms
- GO:0009055 electron carrier activity
- GO:0005624 membrane fraction
- GO:0005789 endoplasmic reticulum membrane
- GO:0055114 oxidation-reduction process
- GO:0006703 estrogen biosynthetic process
- GO:0006805 xenobiotic metabolic process
- GO:0008395 steroid hydroxylase activity
- GO:0019825 oxygen binding
- GO:0020037 heme binding
- View More GO Terms
- GO:0070330 aromatase activity
MCA2077S
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Human ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up