Measles Virus


Native Measles Virus
- Product Type
- Antigen
- Specificity
- Measles Virus
| Native Measles Virus preparation contains a high concentration of virus and viral components. The preparation also contains some cellular material suspended in tissue culture medium. The Measles virus is a highly contagious single-stranded RNA virus that is mostly spread via the respiratory system. It may be passed via aerosol droplets from coughs or through contact with infected bodily fluids. It causes measles, a disease characterised by fever, cough, runny nose, red eyes and a rash. Most patients with uncomplicated measles will recover without antiviral treatment, however, some patients may develop diarrhoea, corneal ulceration, pneumonia or encephalitis. Complications are more likely in adults. In developed countries, most children are immunised against measles by the age of 18 months, as part of the three-part MMR (measles, mumps and rubella) vaccine. |
- Target Species
- Viral
- Product Form
- Inactivated Measles virus - liquid
- Preparation
- Measles virus, Edmonston strain, cultured in Vero cells. Optimally infected cells are disrupted in culture fluids. The suspension is clarified and concentrated by crossflow ultrafiltration. The antigen preparation is inactivated using gamma radiation, which primarily damages viral genetic material.
- Buffer Solution
- Minimum Essential Medium
- Preservative Stabilisers
- None present
- Activity
- Antigenic activity is 104% of internal reference standard.
- Approx. Protein Concentrations
- Current, batch-specific concentration 2.3 mg/ml
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Storage in frost-free freezers is not recommended.
This product should be stored undiluted. Avoid repeated freezing and thawing as this may denature the protein.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| ELISA |
- Instructions For Use
- PIP013 should be sonicated immediately before use to ensure the preparation is uniform. The product may be used in a variety of immunoassay formats or may be further purified to meet the requirements of a particular assay format.
References for Measles Virus
-
Bhoj, V.G. et al. (2016) Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy.
Blood. 128 (3): 360-70. -
Böröcz, K. et al. (2022) Dynamic Features of Herd Immunity: Similarities in Age-Specific Anti-Measles Seroprevalence Data between Two Countries of Different Epidemiological History.
J Clin Med. 11(4):1145. -
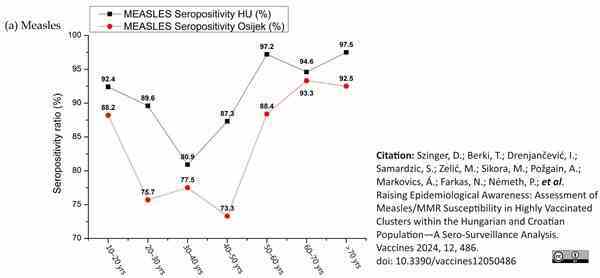
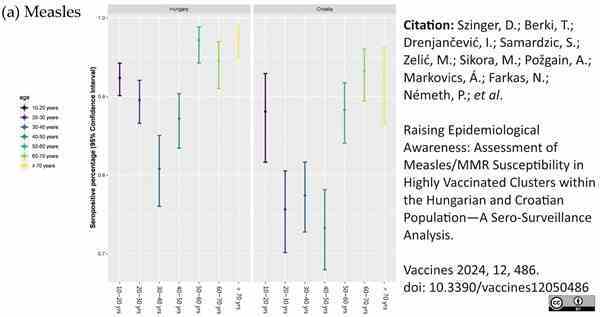
Szinger, D. et al. (2024) Raising Epidemiological Awareness: Assessment of Measles/MMR Susceptibility in Highly Vaccinated Clusters within the Hungarian and Croatian Population—A Sero-Surveillance Analysis
Vaccines. 12 (5): 486.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Viral ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up