
Popular topics

-
References
Collins JJ et al. (2007). The measurement and analysis of age-related changes in Caenorhabditis elegans WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.137.1.
de Cabo R et al. (2014). The search for antiaging interventions: from elixirs to fasting regimens. Cell 157, 1515-1526. https://doi.org/10.1016/j.cell.2014.05.031
Fontana L et al. (2010). Extending healthy life span-from yeast to humans. Science 328, 321-326.
Harrison DE et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392-395.
Kaeberlein M et al. (2005). Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193-1196.
Kennedy BK et al. (2014). Geroscience: linking aging to chronic disease. Cell 159, 709-713.
Kenyon C (2011). The first long-lived mutants: discovery of the insulin/IGF-1 pathway for ageing. Philos Trans R Soc Lond B Biol Sci 366, 9-16.
López-Otín C et al. (2013). The hallmarks of aging. Cell 153, 1194-1217.
Longo VD et al. (2015). Interventions to slow aging in humans: are we ready? Aging Cell 14, 497-510.
Mack HID et al. (2018). The nematode Caenorhabditis elegans as a model for aging research. Drug Discovery Today: Disease Models 27, 3-13.
Tissenbaum HA (2015). Using C. elegans for aging research. Invertebrate reproduction & development 59, 59-63.
World Health Organization (WHO). (2020). Life expectancy. Global Health Observatory (GHO) data. https://www.who.int/gho/mortality_burden_disease/life_tables/situation_trends_text/en/ Accessed on 09/14/20.
An Age Old Question
Shermaine Thein is a 3rd year graduate student at the Centre for Healthy Longevity at the National University of Singapore. Her PhD project is on the utility of novel or established compounds on aging and age-related conditions, using model organisms, like the nematode worm, C. elegans.
In this blog, she shares some of the insights that she has learned about aging as a graduate student stepping into the realm of geroscience.
“How do I stay young?” is a common question I’m asked whenever I explain my research to friends and family. Since the start of time, people have been fascinated by the prospect of living longer and staying young, with the pursuit for eternal youth and the search for a “fountain of youth” a frequent topic in myths and legends. To both the public and researchers alike, aging remains a relatable yet enigmatic subject. Why do we age? And why do some people age differently from others?
Unless you’re a 5000-year old Great Basin bristlecone pine tree, the lifespan of an average human is around 72 years of age (WHO 2020), with women living slightly longer than men. While most of us are well acquainted with the cosmetic and physical aspects of aging like that horrifying new strand of white hair that just sprouted, or more seriously, a decline in muscle function, researchers in the field are delving much deeper to uncover cellular and biochemical processes that drive aging.
Hence, the field of aging attempts to answer rousing questions which are pertinent to our very existence. Importantly, discoveries could mean monumental changes on how we view aging and spur the development of therapeutics to help people live healthier and longer lives.
Aging Is a Complicated Issue
This may be arguably relevant for many conditions, but yes, aging is a really complicated process. Instead of being governed by single pathway, it was actually found to be influenced by nine major processes! These processes were aptly deemed “the hallmarks of aging” by López-Otín et al. These nine hallmarks ranged from stem cell exhaustion and altered intercellular communication to mitochondrial dysfunction and deregulated nutrient sensing (López-Otín et al. 2013). Together, these processes cause the initiation and accumulation of damage in the body over time; ultimately leading to an aging phenotype. Adding further complexity to the mix, the hallmarks were discovered to be closely linked, with changes in one process potentially impinging on others.
Nonetheless, the characterization of these processes continues to provide researchers a comprehensive guideline for future studies. Evidence from independent research groups have shown profound impact in aging when these processes were modulated experimentally. For example, calorie restriction, a well-regarding intervention, extends the lifespan of yeast, worms, and mice due its effects on nutrient sensing pathways (de Cabo et al. 2014). Delineating the contribution of each pathway and their connection with each other remains a challenging yet fundamental question for the field.
Different Species, Similar Pathways
Interestingly, studies have shown commonalities in the pathways influencing aging. In yeast, worms, flies, and mammals, nutrient sensing pathways such as the insulin-like/signaling (ILS) and the mechanistic target of rapamycin (mTOR) pathway are consistently associated with aging. Again, this is illuminated by the conserved effect of dietary restriction, which inhibits both ILS and mTOR, promoting pro-survival effects like improved stress resistance and autophagy across species (Fontana et al. 2010).
Originally discovered in soil bacteria, rapamycin is another prime example of an intervention which inhibits a conserved aging pathway, namely, mTOR. As a result, rapamycin has displayed great success in extending lifespan across species. Feeding 600-day old mice (akin to human middle age) caused a 9-14% lifespan extension, depending on the gender (Harrison et al. 2009). These findings are supported by studies in several other organisms, all of which show rapamycin-induced lifespan extension (Fontana et al. 2010, Kaeberlein et al. 2005). Such cases underscore the conserved nature of aging pathways, and could be valuable for translating discoveries made in model organisms to humans. Promisingly, dietary restriction elicits beneficial effects in people, protecting against common age-related conditions such as cardiovascular disease and insulin resistance (Longo et al. 2015).
Worming Out the Secrets of Aging
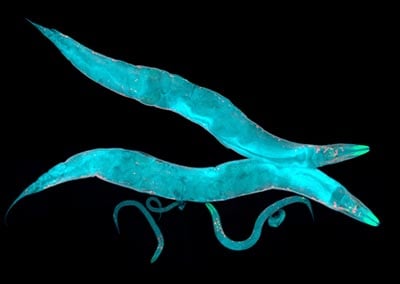
Aging research has advanced greatly with the help of simpler organisms such as worms. As my time in the lab has progressed, my research has become increasingly intertwined with the fate of a tiny worm! The nematode C. elegans has a short lifespan of around three weeks, making it an ideal candidate for observing lifespan changes in a controlled setting, and it is feasible to observe demonstrable life-span changes within the time frame of a PhD (Collins et al. 2007). Moreover, these transparent worms exhibit characteristic features which are quantifiable as they age, such as slower movement and halted reproduction (Mack et al. 2018). They are formidable models, despite having just 959 somatic cells, they give rise to multiple tissues such as a nervous system and even a digestive tract which are also susceptible to age-related decline; just like their mammalian counterparts (Collins et al. 2007). The identification of long-lived strains like daf-2 and age-1 mutants highlighted the role of genetic factors in lifespan extension (Tissenbaum et al. 2015). Screening for pharmacological treatments is also more straightforward in C. elegans, as both healthspan and lifespan effects can be observed in a large cohort of age-synchronized worms.
Hence, contrary to their small size, they have played big roles in aging discoveries and were crucial in identifying not only the first lifespan extension pathway (Kenyon 2011), but also hundreds of signaling pathways and genes responsible for aging!
Helping People Live Healthier for Longer
Modernization has brought about basic comforts such as healthcare, sanitization, and an abundance of food. It’s no surprise then, that we are living longer than our predecessors, who would have been considered fortunate to live past their 50s. Alarmingly, this trend in increasing life expectancy has not been accompanied by an improvement in general health. Studies show that age-related comorbidities such diabetes and cardiovascular disease have increased greatly, with more people living their extended years in poor health (Longo et al. 2015).
Therefore, now more than ever, the pinnacle of aging research has evolved from interventions that extend lifespan, to those that bring about an extension in healthy life expectancy (Kennedy et al. 2014). Simply put, the goal is for people to not merely live longer, but to live healthier for longer.
It is an exciting time to be involved in aging research. The invaluable breakthroughs of the past decades have set precedence for potential interventions that could mediate lifespan and health. As I enthusiastically tackle my own research questions; albeit with the occasional setback, I’m filled with optimism that future findings will bring us a step closer to longer, healthier lives in humans. Perhaps then, I can answer with greater certainty when people ask me “how do I stay young?”
Want to Know More about mTOR Signaling?
Inhibition of the mTOR pathway is relevant for cancer, obesity, diabetes, and even aging. Uncover the proteins involved in our poster
Download the Free Poster
References
Collins JJ et al. (2007). The measurement and analysis of age-related changes in Caenorhabditis elegans WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.137.1.
de Cabo R et al. (2014). The search for antiaging interventions: from elixirs to fasting regimens. Cell 157, 1515-1526. https://doi.org/10.1016/j.cell.2014.05.031
Fontana L et al. (2010). Extending healthy life span-from yeast to humans. Science 328, 321-326.
Harrison DE et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392-395.
Kaeberlein M et al. (2005). Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310, 1193-1196.
Kennedy BK et al. (2014). Geroscience: linking aging to chronic disease. Cell 159, 709-713.
Kenyon C (2011). The first long-lived mutants: discovery of the insulin/IGF-1 pathway for ageing. Philos Trans R Soc Lond B Biol Sci 366, 9-16.
López-Otín C et al. (2013). The hallmarks of aging. Cell 153, 1194-1217.
Longo VD et al. (2015). Interventions to slow aging in humans: are we ready? Aging Cell 14, 497-510.
Mack HID et al. (2018). The nematode Caenorhabditis elegans as a model for aging research. Drug Discovery Today: Disease Models 27, 3-13.
Tissenbaum HA (2015). Using C. elegans for aging research. Invertebrate reproduction & development 59, 59-63.
World Health Organization (WHO). (2020). Life expectancy. Global Health Observatory (GHO) data. https://www.who.int/gho/mortality_burden_disease/life_tables/situation_trends_text/en/ Accessed on 09/14/20.
You may also be interested in...

View more Guest Blog or Feature blogs















