Popular topics

How to detect your target protein or protein complex without interfering IgG chains

Immunoprecipitation (IP) is a key technique for studying protein-protein interactions and isolating individual proteins or protein complexes. A common problem encountered when detecting immunoprecipitates (or immunoprecipitated proteins) by Western blot analysis is the masking of target proteins by immunoglobulins present in the sample or from the antibody bound to the IP beads.
In most cases this will be an immunoglobulin G antibody (IgG), whose denaturing prior to loading onto SDS page results in fragmentation of the IgG antibody into IgG heavy and light chains (~50 and ~25 kDa, respectively). The conventional (heavy and light chain) secondary antibody used for Western blot detection will always detect both IgG chains.
If your target protein is around the same molecular weight as these denatured IgG fragments then they can mask detection of your protein of interest leading to inaccurate conclusions or missing out on detecting potential interaction partners. In addition, if you are performing Western blot analyses of tissue samples such as the spleen, brain or tumors, the presence of high levels of endogenous IgGs in tissues can lead to high background from non-specific secondary antibody binding. Because accuracy and sensitivity are critical for biomedical discovery and basic research, solving these issues is important to all researchers. Several solutions have been proposed (Table 1), however they have major limitations, and only mitigate certain aspects of the obstruction problem. Which raises the question, rather than finding ways to cope with protein masking, what if there was a way to avoid it all together?
TidyBlot™ is a brand new product from Bio-Rad that serves as the perfect solution for avoiding interfering IgGs that mask your target protein(s). It is an HRP conjugated secondary detection reagent that specifically binds to native non-denatured antibodies. According to our R&D team, “TidyBlot gives you confidence that you will be able to detect your protein of interest without interference from IgG fragments from endogenous immunoglobulins or from the antibody used for the immunoprecipitation assay. TidyBlot is also able to detect primary antibodies from a range of host species, enabling researchers to reduce the number of secondary reagents they require.”
Cause of Protein Masking |
Common Proposed Solutions |
Major Limitations |
Why TidyBlot is Better |
|---|---|---|---|
|
Secondary antibody binding to denatured IgGs |
Perform IP under non-reducing conditions without heat such that the antibody complex is then detected at 160 kDa, thus allowing visualization of target proteins. |
The antigen may not effectively be released from the antibody under non-reducing conditions and without heat. |
No need to alter your IP protocol to non-reducing conditions. TidyBlot will not detect the reduced IgG fragments. |
|
Perform Western blot detection using antibodies from different species to avoid cross-reactivity. |
Extra expense and such antibodies might not be readily available. |
No need to purchase alternative antibodies. TidyBlot can detect primary antibodies from a wide range of species and produces background free blots. |
|
|
Use light and heavy chain specific secondary antibodies. Such that when you use a light chain specific antibody the heavy chain will not be detected and vice versa. |
Might miss out on identifying potential protein interactions if there is an interacting protein of the same weight as the other IgG chain. |
TidyBlot only detects native non-denatured antibodies; therefore it avoids interference by denatured antibodies (heavy and light chains). |
|
|
Secondary antibody binding to endogenous IgGs present in tissue samples |
Use a strong blocking buffer that will reduce non-specific binding. |
This may only reduce the general degree of background noise but not specific background from the secondary antibody binding to IgG light and heavy chains. Also the strong blocking could potentially reduce the signal strength. |
No need to purchase and trial expensive blocking buffers. TidyBlot repeatedly produces clean background free blots with inexpensive blocking reagents such as 5% non-fat milk. |
|
Use a species-specific cross-adsorbed secondary antibody that does not cross-react with the target protein species. |
Such an antibody may not be available, depending on your protein of interest. |
No need to purchase multiple cross-adsorbed secondary antibodies, as TidyBlot detects primary antibodies from a wide range of species with high sensitivity. |
Table 1. Common solutions for managing interfering IgGs in IP and Western blot experiments and their limitations
Eliminate background with TidyBlot
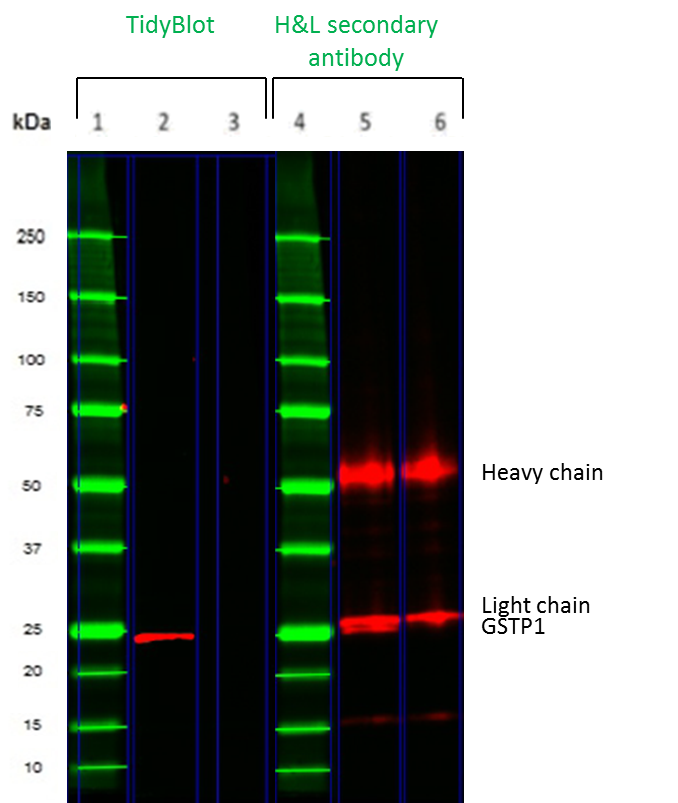
Fig.1. Comparison of TidyBlot and a conventional secondary antibody for detection of the 50kDa GSTP1 protein in a mock IP experiment
The proof is in the results as we have tested TidyBlot extensively to demonstrate that it eliminates problems caused by interference from denatured IgGs.
In Figure 1, we compare TidyBlot with a conventional secondary antibody in Western blot detection of a mock IP. K562 leukemia cell lysates were spiked with a mouse monoclonal antibody of the IgG2b isotype prior to SDS-PAGE under reducing conditions. Glutathione S-transferase P (GSTP1), which has a molecular weight of ~25 kDa, was detected with TidyBlot (lane 2) or an HRP conjugated goat anti-mouse (heavy and light chain) secondary antibody. When a conventional secondary antibody was used (lanes 5 and 6), heavy and light chain bands were detected. The light chain masks the GSTP1 protein (lane 5). When TidyBlot was used (lane 2), no heavy and light chain bands were observed and GSTP1 was accurately and unambiguously detected. Lanes 3 and 6 are no primary antibody controls; these controls were used to determine the background caused by secondary detection reagents.
We also tested the species compatibility of TidyBlot and show that it can detect primary antibodies raised in a number of different species, including goat, rabbit and sheep (Figure 2). However, TidyBlot has one limitation in that it demonstrates variable affinity for mouse IgG1 antibodies. We therefore suggest performing dot blot analysis to determine compatibility in such instances.
Alternatively, if you are interested in detecting a protein of ~50 kDa with a mouse primary antibody, Bio-Rad offers an HRP conjugated rat anti-mouse kappa light chain antibody (OX-20) (MCA152P). This light chain specific secondary antibody will not detect mouse IgG heavy chains, thus allowing unhindered detection of your 50 kDa protein. More information about TidyBlot’s compatibility can be found here.
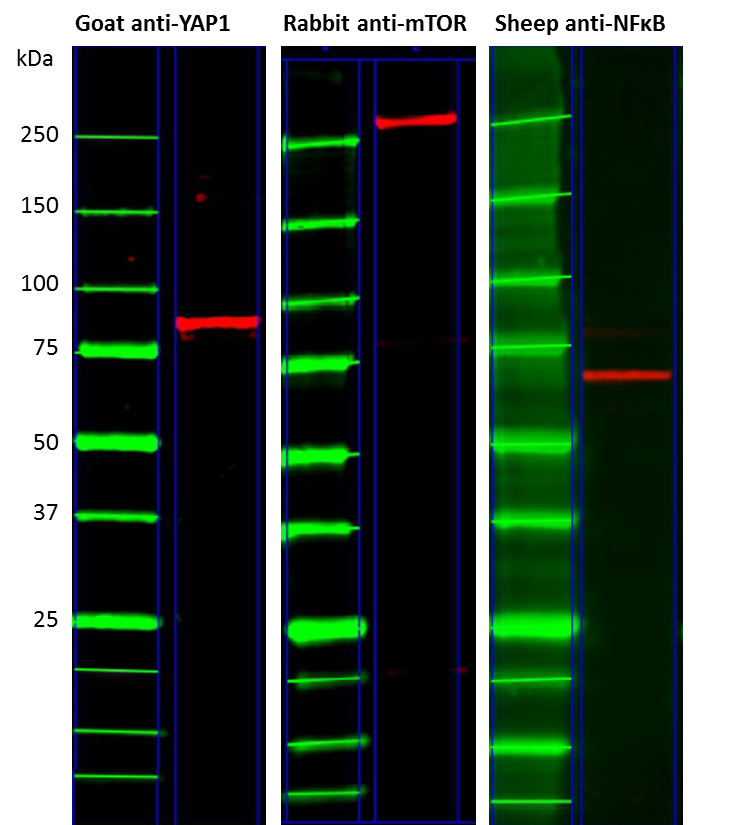
Fig.2. Broad species compatibility of the TidyBlot reagent
No need to adapt your existing protocol
Perhaps the best thing about TidyBlot is that you do not need to change your IP or Western blot protocol in order to produce background-free publication-ready results. All you need to do is simply swap your conventional HRP conjugated secondary antibody for TidyBlot.
In addition, TidyBlot gives you confidence in your results and conclusions and ensures that you won’t miss out on making new discoveries by not identifying all proteins in a protein complex.
Learn more about TidyBlot
See what others are saying about TidyBlot in our customer reviews section.
Also check out our TidyBlot FAQ page for quick answers to the most common questions about using TidyBlot.
You may also be interested in...

View more Applications or Western Blotting blogs