
Popular topics

-
References
Venkadakrishnan VB et al. (2019). Protein Kinase N1 control of androgen-responsive serum response factor action provides rationale for novel prostate cancer treatment strategy. Oncogene 38, 4496-4511.
Slash the Tires Instead to Slow Down Prostate Cancer
2020 Bio-Rad Science Writing Competition 3rd Place
Varadha Balaji is a 5th year grad. student enrolled in the Regulatory Biology program at Cleveland State University.
He impressed the judges with his clever race car analogy to explain the biology of prostate cancer. His passion for his research was evident throughout his piece and it scored highly for originality and writing style.
We are delighted to publish his entry below.
One in every nine American men will be diagnosed with prostate cancer, making it the most frequently diagnosed cancer among western men. This year alone, we expect prostate cancer to kill over 33,000 Americans. Why is it such a hard disease to treat?
Let me explain the biology of prostate cancer with a race car analogy (Figure 1). If prostate cancer is like a dangerous supersonic car, then the high power engine inside that car is a molecular engine called the androgen receptor. The fuel for this engine is the male hormone testosterone. One can think of the exhaust, when this engine is running, as prostate specific antigen (PSA). PSA is an androgen receptor target gene which makes it a very suitable biomarker for prostate cancer diagnosis and monitoring disease progression.
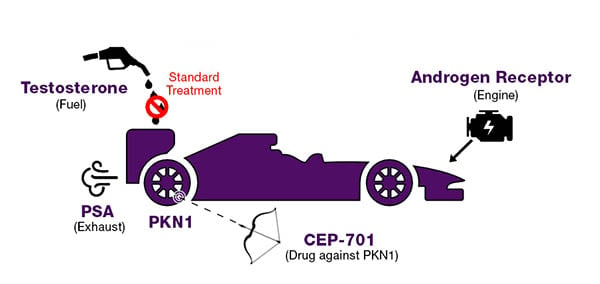
Fig. 1. Prostate cancer race car analogy. The male hormone testosterone powers prostate cancer’s engine (the androgen receptor), like fuel powers a race car. PSA acts like the exhaust and can be used as a marker. Drugs like PKN1 can be used to slash the tires and stop the car, for instance, as a targeted cancer treatment.
The goal for researchers has been focused on ways to stop this car. If prostate cancer is a race car, then shutting down the engine should make this car come to a complete halt. Getting rid of the fuel, such as testosterone, should stop the engine from running. This is the basic idea of androgen deprivation therapy, the standard treatment for metastatic prostate cancer. This “castration” therapy reduces the disease burden of the patient for a few years. However, prostate cancer develops resistance to this therapy and progresses on to a state called castration-resistant prostate cancer, or CRPC. It appears as if prostate cancer goes into a pit-stop, gets re-engineered, and finds a number of ways to re-activate the engine, even without testosterone. For the last 80 years, we have been developing and throwing multiple drugs at the androgen receptor, trying our best to jam this engine - yet it always finds ways to keep itself running which ultimately leads to the death of the patient.
Our laboratory is trying a different approach to stop this car, shifting our focus from the engine. While the engine keeps running, is it possible to slow down this car by taking out its tires? This is easier said than done because prostate cancer is clever at hiding its tires as well. First, we have to identify the tires and second, these tires have to be targetable.
In my dissertation research, I performed a screening experiment to locate at least one of the tires. I identified protein kinase N1 (PKN1) as the mediator of a critical signaling pathway downstream of activated androgen receptor. PKN1 is over-expressed in CRPC as compared to treatment-naïve prostate cancer or benign. In preclinical mouse models, overexpression of PKN1 led to increased tumor growth under treatment-naïve conditions. Upon castration, animals bearing PKN1 over-expressing tumors experienced poorer survival. Gene silencing of PKN1 led to a reduction in cell viability and in vitro and site-directed mutagenesis experiments indicated the significance of kinase function of PKN1. These results made PKN1 an attractive target, which potentially could be blocked using a kinase inhibitor. With PKN1 as the tire to be targeted, we sought to look for the most suitable way to take it out.
CEP-701 is a multi-kinase inhibitor that has been reported to block PKN1 activity. Using RNA sequencing, I demonstrated that CEP-701 could block PKN1 with appreciable accuracy. The reduction in cell proliferation and altered gene expression when treated with CEP-701 was comparable to the effects observed with PKN1 silencing. CEP-701 treatment showed favorable effects on tumor growth in preclinical models. However, there was one problem: CEP-701 has already been tested in prostate cancer clinical trials. The primary clinical end point that was used in this trial was a 50% reduction in PSA levels. Unfortunately, CEP-701 failed to achieve this predetermined clinical end point and no patients enrolled in this trial reached a 50% reduction in PSA level. As a result, the trial was terminated and CEP-701 was not investigated further.
PSA is an indicator of activated androgen receptor; the exhaust that’s pumped out when the engine starts running. CEP-701 targets PKN1, the tire in this analogy. Therefore, how would slashing the tires lead to a difference in how much exhaust is pumped out of the car? Taking out a tire may slow down a car, but it will not result in a 50% reduction in exhaust pumped out. We need to identify an appropriate biomarker to monitor the treatment response of CEP-701. Delineating PKN1 signaling is essential for the development of an accurate biomarker to monitor PKN1 inhibition. I am currently setting up experiments to more comprehensively understand the biology of PKN1.
I am a fifth year grad student projected to defend soon and this work has been published recently in Oncogene (Venkadakrishnan et al. 2019). It has been a wonderful, adventurous journey and I look forward to discussions in the future with the person who would pursue this project in our lab. The next set of experiments will hopefully reveal an accurate biomarker to monitor PKN1 inhibition which will result in a new strategy to stop this car.
Bio-Rad's Science Writing Competition Results
There was a huge number of entries from all around the world and the judges were impressed by the high standard of submitted articles across a breadth of different topics. Varadha Balaji placed 3rd in the competition and received a commemorative certificate to mark this achievement.
References
Venkadakrishnan VB et al. (2019). Protein Kinase N1 control of androgen-responsive serum response factor action provides rationale for novel prostate cancer treatment strategy. Oncogene 38, 4496-4511.
You may also be interested in...
















