
Popular topics

-
References
Knappik A et al. (2000). Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol 296, 57-86.
Nash D et al. (2001). The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 344, 1807-1814.
Shiryaev S et al. (2010). Isolation and characterization of selective and potent human Fab inhibitors directed to the active site region of the two-component NS2B-NS3 proteinase of West Nile virus. Biochem J 427, 369-376.
Engineering recombinant inhibitory antibodies as research tools
The West Nile virus (WNV) was first detected in 1937 in the West Nile District of Uganda. It was isolated from a 37 year old woman living in the region during a research study on Yellow Fever. Similar to the virus that causes Yellow Fever, WNV is a mosquito-borne virus of the Flavivirus genus.
WNV did not make its way to the western hemisphere until over six decades later during an outbreak in Queens New York in the United States (US) in 1999 (Nash et al. 2001). Between 1999 and 2015, there have been over 40,000 cases of WNV infection and over 1,900 deaths in the US. While WNV infection is asymptomatic in approximately 70-80% of cases, around 20% will develop West Nile fever and another 1% can develop serious neurological diseases such as encephalitis and meningitis.
New treatment strategies
To date, there are no approved treatments or preventative vaccines for WNV infection, but scientists are actively working on strategies to address this. One approach involves the development of inhibitors that block the function or activity of key enzymes in the virus.
Antibodies are excellent scaffolds for designing inhibitors against specific enzymes of a virus, particularly because they can target nearly any antigen. For example, they can distinguish between wild type and mutant forms of a viral enzyme. The design of large recombinant antibody libraries combined with phage display technology enables the use of sophisticated in vitro selection steps that facilitate the isolation of antibodies with such desired characteristics.
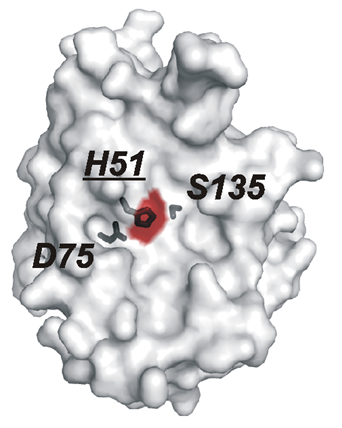
Researchers at the Sanford-Burnham Institute for Medical Research partnered with scientists at Bio-Rad to engineer high affinity, highly specific inhibitor antibodies to the active site region of a WNV proteinase (Shiryaev et al. 2010). Using the synthetic naïve Human Combinatorial Antibody Library (HuCAL®) and CysDisplay®, an innovative method of phage display, Bio-Rad scientists generated selective and potent inhibitor antibodies to the WNV non-structural (NS) protein 2B-NS3 serine proteinase.
This is the only proteinase encoded by the flavivirus genome, and its activity is necessary for virus infectivity. Therefore, therapeutic antibodies that inhibit its function could prove useful for treating or preventing WNV infection.
Our latest technical article describes the guided selection strategies that led to the isolation of antibodies specific to the functional active site of wild-type WNV NS2B-NS3 proteinase. The antibodies generated were highly specific as they did not recognize structurally similar virus proteinases from Dengue virus type 2 or the hepatitis C virus; nor did they inhibit human serine proteinases (Shiryaev et al. 2010).
These highly specific antibodies have been used as reagents for further studies on NS2B-NS3 active site binding and dynamics. More importantly, they are also useful tools for the development of therapies or vaccines against WNV.
References
Knappik A et al. (2000). Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol 296, 57-86.
Nash D et al. (2001). The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 344, 1807-1814.
Shiryaev S et al. (2010). Isolation and characterization of selective and potent human Fab inhibitors directed to the active site region of the two-component NS2B-NS3 proteinase of West Nile virus. Biochem J 427, 369-376.
You may also be interested in...

















