
Popular topics

-
References
Campbell N (2020). First report of human monoclonal antibody that blocks SARS-CoV-2. Technology Networks: https://www.technologynetworks.com/biopharma/news/first-report-of-human-monoclonal-antibody-that-blocks-sars-cov-2-332110. Accessed on 05/13/20
Chames P et al. (2009). Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol 157, 220–233.
Chan CEZ et al. (2009). The use of antibodies in the treatment of infectious diseases. Singapore Med J 50, 663.
Langreth and Berfield. (2020). Antibody treatments may be the best hope against the virus until a vaccine. Bloomberg Businessweek: https://www.bloomberg.com/news/features/2020-04-20/antibody-treatments-may-be-the-best-hope-against-the-virus-until-a-vaccine Accessed on 05/13/20
Millet JK and Whittaker GR (2018). Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology 517, 3-8.
Prabakaran P et al. (2009). Potent Human monoclonal Antibodies against SARS-CoV, Nipah and Hendra Viruses. Expert Opin Biol Ther 9, 355-368.
Salazar G et al. (2017). Antibody therapies for the prevention and treatment of viral infections. NPJ Vaccines 2, 19.
Shanmugaraj B et al. (2020). Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol 38, 10-18.
Shi R et al. (2020). A human neutralizing antibody targets the receptor binding site of SARS-CoV-2. Nature. 462.
Tortorici MA and Veesler D (2019). Structural insights into coronavirus entry. Adv Virus Res 105, 93-116.
Walls AC et al. (2020). Structure, function and antigenicity of the SARs-CoV-2 spike glycoprotein. Cell 180, 1-12.
Walsh et al. (2016). Blood-borne pathogens: a canadian blood services centre for innovation symposium. Transfus Med Rev 30; 53–68.
Wang C et al. (2020). A human monoclonal antibody blocking SARS-CoV-2 infection. Nature Commun 11, 2251.
Wang H et al. (2008). SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Research 18, 290–301.
Could a Monoclonal Antibody Cure COVID-19?
The development of the COVID-19 pandemic has led to research groups racing to identify vaccines and targeted therapeutics to prevent or treat the disease, and to reduce the spread of infection. In this blog, we discuss the potential for a monoclonal antibody to neutralize the SARS-CoV-2 virus that causes COVID-19.
How Are Antibodies Used as Cures?
Antibodies are essential for a healthy immune system, recognizing invading pathogens and communicating to immune cells that a threat needs to be destroyed. The idea that antibodies can be used to cure infectious disease is nothing new. It was back in 1890 that von Behring and Kitasato discovered that sera from immune animals or humans can be protective against infectious diseases (Chan et al. 2009). By the 20th century, serum therapy was routinely used to treat a variety of bacterial infections, but it was often associated with severe side effects. Once antibiotics were developed and their use became widespread, serum therapy dropped out of popularity (Chan et al. 2009).
One problem with serum therapy was that sera from different donors or animals could not be standardized. Two treatments would contain different concentrations or antibodies, meaning their efficacy would not be the same and treatment results would vary (Chan et al. 2009). Sera could also contain undetected blood-borne pathogens, such as hepatitis B, which actively harm the patient (Shanmugaraj et al. 2020; Walsh et al. 2016). The interest in serum therapy for the treatment of disease re-emerged in 1975 when Kohler and Milstein discovered a way to ensure consistency and reduce the risk of blood-borne infection: the efficient production of monoclonal antibodies (Chames et al. 2009).
Monoclonal antibodies are now used as specialist drugs to treat autoimmune and cardiovascular diseases, cancer, and inflammation; over 60 recombinant monoclonal antibodies have been developed for human use in recent years (Salazar et al. 2017). However, antibodies are less frequently used to treat infectious diseases as other treatments, like antibiotics, offer an effective and cheaper option (Langreth and Berfield 2020). Many monoclonal antibodies have been developed against viruses in recent years, and many more are in the pipeline. With increasing concerns over antibiotic resistance, many monoclonal antibodies could soon become a go-to treatment for infectious diseases (Chan et al. 2009).
One current question is in the race to find effective solutions, could a monoclonal antibody be used to cure COVID-19?
How Coronaviruses Enter Host Cells
SARS-CoV-2, the contagion that causes COVID-19 in humans, belongs to a family of viruses known as coronaviruses along with SARS-CoV and MERS-CoV. Both SARS-CoV and SARS-CoV-2 possess a transmembrane spike (S) glycoprotein which forms homotrimers protruding from the surface of the virus (Tortorici and Veesler 2019). This glycoprotein comprises two functional subunits. The S1 subunit is responsible for binding to transmembrane proteins on the surface of a host cell while the S2 subunit fuses the viral membrane to the host cell membrane (Figure 1).
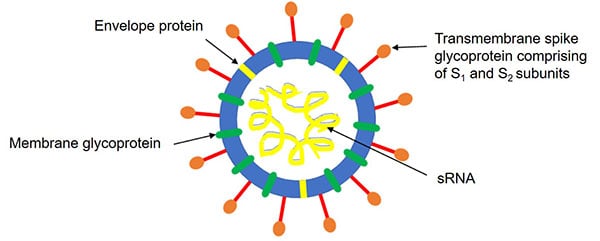
Fig. 1. Illustration of the coronavirus structure.
The S1 subunit of the transmembrane spike glycoprotein on SARS-CoV-2 can bind to the transmembrane protein angiotensin-converting enzyme 2 (ACE2) present on human throat and lung cells with similar affinity to the S1 subunit on SARS-CoV (Walls et al. 2020). The binding of the S1 subunit of SARS-CoV to the enzymatic domain of ACE2 triggers endocytosis and results in the translocation of the virus and the enzyme into the cell (Wang et al. 2008) (Millet and Whittaker 2018). ACE2 may therefore also serve as an entry receptor for SARS-CoV-2 (Walls et al. 2020), leading researchers to explore whether blocking the binding of SARS-CoV-2 to ACE2 with neutralizing antibodies could be a viable strategy for treating COVID-19.
Developing Neutralizing Antibodies against SARS-CoV and SARS-CoV-2
Neutralizing antibodies are generated in response to infection by coronaviruses including SARS-CoV and SARS-CoV-2. They bind to specific antigens on the surface of the virus and prevent the virus from entering a host cell. In the case of SARS-CoV, neutralizing antibodies are generated in response to infection and target the transmembrane S glycoprotein, preventing SARS-CoV from binding to ACE2 and entering the host cell (Prabakaran et al. 2009).
Polyclonal antibodies have previously been generated commercially against SARS-CoV. Experiments conducted by Walls et al. in 2020 showed that polyclonal antibodies produced in mice that target the S protein on SARS-CoV also inhibited SARS-CoV-2 entry into cells. This suggests that antibodies targeting conserved regions on the transmembrane S glycoprotein of both viruses could be generated upon vaccination with the transmembrane S glycoprotein of SARS-CoV.
However, polyclonal antibodies have drawbacks; the most problematic being high lot-to-lot variability and risk of inducing allergic reactions. Recombinant technology enables the production of antibodies that are highly consistent between batches and is often used to design antibody drugs including monoclonal chimeric animal-human, humanized, and fully human antibody formats. It is also easier to determine the mechanisms behind the therapeutic efficacy of monoclonal antibodies and thereby engineer them to improve their therapeutic properties (Prabakaran et al. 2009). Monoclonal antibodies also have low toxicity and high specificity, which makes them valuable for cancer treatment, for example. In contrast with the scatter-gun approaches of chemotherapy and radiotherapy, which can damage healthy tissues, antibodies that have passed clinical trials have demonstrated their ability to directly target the correct antigen with minimal side effects (Chan et al. 2009).
A Monoclonal Antibody That Can Neutralize SARS-CoV-2
Wang et al. (2020) aimed to develop a cross-neutralizing monoclonal antibody that targets a conserved epitope found on the transmembrane S glycoprotein of both SARS-CoV and SARS-CoV-2. ELISA cross-reactivity tests were performed to find chimeric antibodies comprising human variable heavy and light chains and rat constant regions which bound to the S1 subunit of both viruses, and that also displayed cross-neutralizing activity upon infection with vesicular stomatitis virus pseudotyped to express the transmembrane spike glycoprotein of SARS-CoV or SARS-CoV-2. One such chimeric antibody, clone 47D11, was converted into a fully human antibody of IgG1 isotype for further study.
47D11 was shown to bind to HEK293 cells (human embryonic kidney cells) expressing the transmembrane spike glycoprotein of SARS-CoV or SARS-CoV-2, and neutralized SARS-CoV and SARS-CoV-2 infection of VeroE6 cells (African green monkey kidney cells). Surprisingly, 47D11 did not prevent binding of the S1 subunit of the transmembrane spike glycoprotein to the ACE2 receptor on the surface of VeroE6 cells for either virus, suggesting that this antibody neutralizes SARS-CoV and SARS-CoV-2 via a mechanism independent of receptor binding. This may be because 47D11 binds to a conserved region of the S1B subunit of the transmembrane spike glycoprotein that is further away from the receptor-binding interface and is therefore unable to interfere with spike-receptor interaction (Wang et al. 2020).
Although further investigations need to be conducted into the mechanism by which 47D11 neutralizes infection with SARS-CoV and SARS-CoV-2, the results from this study raise the question of whether a monoclonal antibody could be effective against COVID-19 in humans.
Monoclonal Antibodies: a Practical Solution to COVID-19?
While developments in the rush to find a cure are exciting, not enough time has elapsed yet for a positive safety and efficacy profile of an antibody against COVID-19 to be demonstrated in humans. Research lead of the 47D11 study, Berend-Jan Bosch, emphasized that while the development represents a positive first step, it is too early to tell about the antibody’s efficacy in humans (Campbell 2020).
In addition to Wang et al., many other groups are investigating whether existing or new monoclonal antibodies could be used in the fight against COVID-19. So far, several antibodies have demonstrated promising results in vitro and in vivo against SARS-CoV, suggesting that they could be developed into potentially effective therapies (Shanmugaraj et al. 2020). Recently, Shi et al (2020) also reported the isolation of two specific human monoclonal antibodies from a patient who had recovered from COVID-19. One clone in particular, CB6, demonstrated potent neutralizing activity against SARS-CoV-2 in vitro and inhibited SARS-CoV-2 infection in rhesus monkeys.
However, there may be limitations to the production of monoclonal antibodies for therapeutic use. Large-scale production of monoclonal antibodies is expensive, labor-intensive, and time-consuming. There also may be limited global capacity to make monoclonal antibodies in the quantities required for them to become a mainstay of treatment (Langreth and Berfield 2020). However, recent developments in recombinant antibody technology may bring down production costs and timescales, with the possibility to clone in mammalian, yeast, or plant expression systems (Shanmugaraj et al. 2020).
So, will a monoclonal antibody cure COVID-19? We don’t know yet, but we are encouraged by the results so far and are grateful to all the researchers working tirelessly towards finding an effective vaccine.
References
Campbell N (2020). First report of human monoclonal antibody that blocks SARS-CoV-2. Technology Networks: https://www.technologynetworks.com/biopharma/news/first-report-of-human-monoclonal-antibody-that-blocks-sars-cov-2-332110. Accessed on 05/13/20
Chames P et al. (2009). Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol 157, 220–233.
Chan CEZ et al. (2009). The use of antibodies in the treatment of infectious diseases. Singapore Med J 50, 663.
Langreth and Berfield. (2020). Antibody treatments may be the best hope against the virus until a vaccine. Bloomberg Businessweek: https://www.bloomberg.com/news/features/2020-04-20/antibody-treatments-may-be-the-best-hope-against-the-virus-until-a-vaccine Accessed on 05/13/20
Millet JK and Whittaker GR (2018). Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology 517, 3-8.
Prabakaran P et al. (2009). Potent Human monoclonal Antibodies against SARS-CoV, Nipah and Hendra Viruses. Expert Opin Biol Ther 9, 355-368.
Salazar G et al. (2017). Antibody therapies for the prevention and treatment of viral infections. NPJ Vaccines 2, 19.
Shanmugaraj B et al. (2020). Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol 38, 10-18.
Shi R et al. (2020). A human neutralizing antibody targets the receptor binding site of SARS-CoV-2. Nature. 462.
Tortorici MA and Veesler D (2019). Structural insights into coronavirus entry. Adv Virus Res 105, 93-116.
Walls AC et al. (2020). Structure, function and antigenicity of the SARs-CoV-2 spike glycoprotein. Cell 180, 1-12.
Walsh et al. (2016). Blood-borne pathogens: a canadian blood services centre for innovation symposium. Transfus Med Rev 30; 53–68.
Wang C et al. (2020). A human monoclonal antibody blocking SARS-CoV-2 infection. Nature Commun 11, 2251.
Wang H et al. (2008). SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Research 18, 290–301.
You may also be interested in...

View more Science News or Article blogs















