
Popular topics

Take a quantum leap in your IF experiments with our 5 tips and tricks
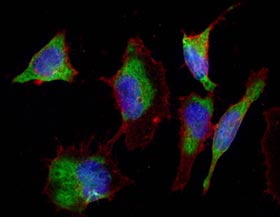
These five tips are designed to assist you with the selection of fluorophores for your imaging experiments.
1. Familarize yourself with your microscope set up (especially with the emission/excitation/dichroic filters and in the case of confocal microscopes the laser lines). Knowing your set-up ensures that you can select fluorophores that can be optimally excited and detected.
2. To assess fluorophore/microscope compatibility we recommend you consult a spectrum viewer or a fluorophore reference chart. These references help to determine the maximum excitation and emission wavelengths of the fluorophore conjugated antibodies present in your lab. Compatibility evaluation can be straight forward for certain filters, such as FITC/TRITC filters, as these have been named after the fluorophores they are meant to be used with. As a rough guideline a FITC filter is suitable for most green emitting fluorophores including new generation dyes, such as Alexa Fluor® 488. As members of dye families, such as Alexa Fluor® 488, have been named/numbered according to their approximate excitation maxima (in nm), these numbers also provide a rough guidance for laser and filter selection.
3. Select fluorophores with high extinction coefficients ( ε ). One defining factor of a fluorophore’s brightness is its extinction coefficient (a measurement of the probability of absorbing a photon of light); the higher the value of the extinction coefficient the brighter the fluorophore. For example the rather dim DyLight® Fluor 350 has an extinction coefficient of only 15,000 while the bright DyLight® Fluor 650 dye has an extinction coefficient of 250,000 (Thermo Fisher Scientific Inc 2012).
4. Choose fluorophores with high quantum yields (the quantum yield is a read-out of the efficiency of the fluorescence process; Φ is calculated by dividing the number of emitted photons by the number of absorbed photons). Based on the nature of the equation a 100% efficient fluorescence process would have a quantum yield of 1 (the maximum quantum yield possible). The commonly used and very bright Alexa Fluor® 488 fluorophore for example has a high quantum yield of 0.92 (Molecular Probes® 2010).
5. Avoid fluorophores with a high susceptibility to photobleaching (a photochemical destruction process which reduces the intensity of the fluorescence signal; for example FITC and R-Phycoerythrin are known to photobleach quickly). To reduce fading of the fluorescence signal due to photobleaching we recommend you use photostable fluorophores, such as Alexa Fluor® or DyLight® Fluor dyes. Alternative options to minimize photobleaching are to reduce the intensity/exposure time to the excitation light and to use mounting media containing antifade reagents. For more information about mounting media, please refer to the Mounting coverslip section of the Bio-Rad IHC-P tips and tricks ebook.
References
Thermo Scientific Inc (2012). Thermo Scientific Pierce Fluorescent Products Guide.
Available here (Accessed: May 13, 2015).
Molecular Probes® (2010). Molecular Probes Handbook, A Guide to Fluorescent Probes and Labeling Technologies.
Available here (Accessed: May 13, 2015).
You may also be interested in...

View more Applications or Immunofluorescence blogs















