Article: An Accelerated Approach to Sensitive ADA Assays
-
Monoclonal Generation
-
Custom Recombinant Monoclonal Antibody Generation
-
Webinars, Videos and Technical Articles
- Webinar: Overcome the Challenges of PK Assay Development Using TrailBlazer Antibodies
- Webinar: Generation of SARS-CoV-2 antibodies in multiple formats within four weeks
- Webinar: Recombinant Antibodies with SpyTag Technology
- Webinar: Transform bioanalytical assays with TrailBlazer Antibodies
- Webinar: Control your critical antibody reagents and avoid assay failure
- Webinar: Improve your antibody drug development assays
- Webinar: Optimize your assays using recombinant antibodies selected for desired affinity
- Webinar: The making of recombinant anti-idiotypic antibodies for high performance in bioanalytical assays
- Webinar: Human recombinant antibodies as positive controls and calibrators
- Webinar: How to overcome assay challenges using custom recombinant antibodies
- Webinar: Generation of high affinity recombinant antibodies for application in immuno-MRM
- Video: Generating anti-idiotypic antibodies for bioanalytical assays
- Video: Best practices for characterization and QC of anti-idiotypic antibodies for bioanalysis
- Video: Generation of drug-target complex specific antibodies
- Video: Antibodies for CAR-T cell therapy development
- Article: Monitoring antibody immune responses against biotherapeutic drugs
- Article: Effective tools for drug monitoring assays
- Article: An accelerated approach to sensitive ADA assays
- Article: Isolation of enzyme active site-specific recombinant antibodies by guided selection
- Article: Biomarker Assay Development using Highly Specific Recombinant Antibodies
- Article: Recombinant antibodies as standards for immunodiagnostic assays
- Article: High affinity antibodies for peptide enrichment immuno-MRM
- Article: Generation of antibodies against self-antigens
- Article: Generation and characterization of drug-target complex-specific antibodies
- Article: Antibodies for CAR-T Cell Therapy Development
- Poster: Faster Generation of Anti-Drug Antibodies Using SpyTag Technology
- Poster: CAR T cell analysis with modular antibodies
- Poster: Drug-Target-Complex Specific Antibodies for Pharmacokinetic Analysis of Biotherapeutics
- Poster: Characterization of anti-idiotypic antibodies for high performance in bioanalytical assays
- Poster: Recombinant anti-idiotypic antibodies for antibody drug development
- Poster: Generation of recombinant antibodies for Bio-Plex assays
-
Webinars, Videos and Technical Articles
-
Custom Recombinant Monoclonal Antibody Generation
s
Simplified sourcing via Scientist.com
s
Custom Antibody Project Inquiry Form
A personal, no obligation quotation for a custom monoclonal antibody generation project
s
Contact our custom antibody specialists
Tel: +49 (0) 89 80 90 95 45
Fax: +49 (0) 89 80 90 95 50
Office: Bio-Rad AbD Serotec GmbH, Campus Neuried, Anna-Sigmund-Str. 5, 82061 Neuried, Germany
A Faster Route to More Sensitive Assays for the Monitoring of Drug Antibodies
Monitoring of immune responses caused by biotherapeutic drug treatment is a regulatory requirement during preclinical development through to clinical trials. Optimization and validation of assays for anti-drug antibodies (ADA) is challenging, particularly when the drug itself is a human monoclonal antibody due to the excess of human antibodies in serum and the relatively low immune response. Highly specific and sensitive detection antibodies are needed for robust pharmacokinetic (PK) and anti-drug antibody (ADA) assays.
Binding Strength Matters
The performance of an antibody in an assay often correlates with its affinity to the antigen. The ability to specifically select for antibodies on the basis of binding strength is therefore an important element of antibody assay development. Affinity or koff-rate determination can be carried out on several platforms; for example a rough ranking by ELISA, or quantitative screening using a technology such as surface plasmon resonance (SPR) or biolayer interferometry (BLI).
Recombinant monoclonal antibodies against biotherapeutics can be created using sophisticated antibody libraries and well-established phage display technology. Such methods overcome the unpredictability and inflexibility of traditional hybridoma technology, and the resulting antibodies offer significant advantages for generating highly specific and sensitive assays for the drug development process.
High-throughput recombinant antibody generation using HuCAL® technology (Figure 1) results in about 100-200 unique antibodies confirmed ELISA-positive after the Primary Screening process. Typically only a smaller fraction of these are further analyzed, since the subsequent characterization of individual purified antibodies in various assays can be a rate-limiting step.
Knowing the binding strength of ELISA-positive tested antibodies would enable a more educated clone selection, and improve the chances of selecting the best antibodies for the bioanalytical assay development.
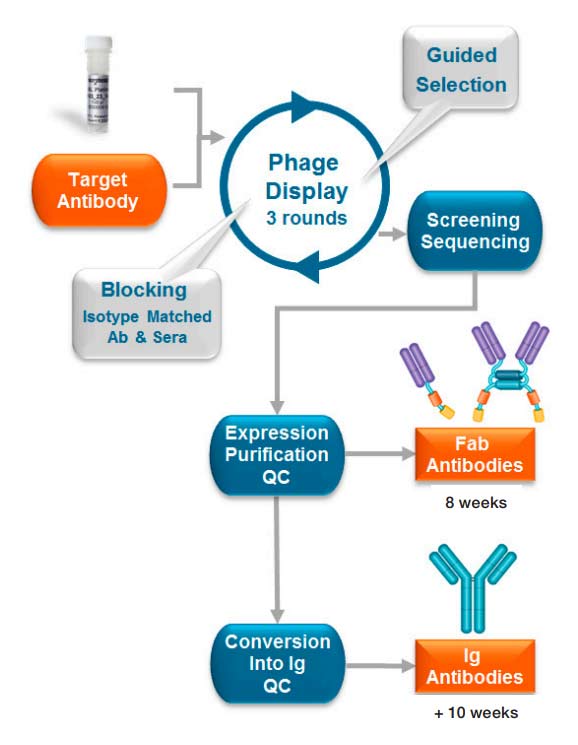
Fig. 1. Anti-idiotypic antibody process using HuCAL technology.
High-throughput Off-Rate Screening
Ylera and colleagues (Ylera et al. 2013) developed an additional high throughput screening step that allows selection of antibodies according to their koff-rate and applied it to the generation of anti-idiotypic antibodies against the antibody drugs cetuximab and trastuzumab.
Antibodies binding to cetuximab and trastuzumab were selected from the HuCAL PLATINUM® phage-display library, and one of the anti-trastuzumab antibodies underwent further affinity maturation to generate ultra-high affinity antibodies. The antibody koff-rate determination was incorporated into the high-throughput process as secondary screening.
Fully human, recombinant Fab antibodies were expressed in E. coli and their interaction kinetics with the respective drug antibody were measured by biolayer interferometry (BLI). The label-free sensor instrument used allowed accurate determination of antibody koff-rates in crude bacterial lysates. Use of crude lysates to produce antibodies is fast, simple and inexpensive, and therefore most suitable for screening steps. The koff-rate values measured using crude bacterial lysates in secondary screening correlated well with the data obtained by affinity measurement of purified antibodies in confirmatory experiments.
For the anti-cetuximab and both anti-trastuzumab projects, koffrate values of antibodies were determined and antibodies with the lowest koff-rates identified; this demonstrated that the method is also suitable for antibodies obtained from affinity maturation projects which often exhibit subnanomolar affinities. The matured anti-trastuzumab antibody ranked as having the highest affinity was compared with the parental antibody in a bridging ELISA format and the data highlighted the difference in sensitivity obtained with the affinity matured antibody (Figure 2).
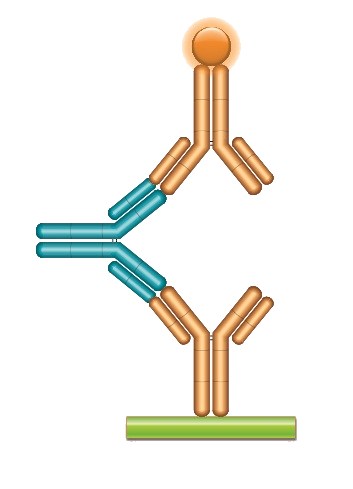
Fig. 2a. A typical ADA bridging assay format using a fully human monoclonal anti-idiotypic antibody as positive control or reference standard.
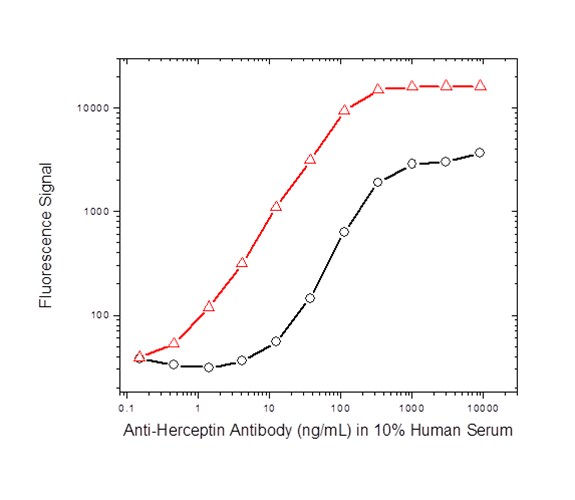
Fig. 2b. ADA assay with anti-trastuzumab antibodies: affinity matured AbD18018 (KD = 65 pM, red); parental antibody AbD16714 (KD = 2.5 nM, black); both in the IgG1 format as shown in Fig. 2a.
Faster Development of Sensitive Assays
The ranking of antibodies according to their binding strength early in the antibody generation process confers the ability to select clones with favorable binding properties prior to further characterization and assay development. The consequence for the assay developer is a faster route to more sensitive assays for the monitoring of drug antibodies.
References
-
F. Ylera et al. 2013. Off-rate screening for selection of high-affinity anti-drug antibodies.
Anal. Biochem 441(2):208-213






