Popular topics

ELISAs Do Little? Your Guide to ELISAs Part One
Enzyme-Linked Immunosorbent Assays (or ELISAs) are widely used for the detection and quantification of proteins, peptides, hormones, and other substances in a sample. ELISAs are relatively cheap to run, highly reproducible, and require comparatively little hands on time. They demonstrate higher sensitivity than other applications like western blot, and are able to detect picogram amounts of the target antigen.
The inclusion of standards with known quantities of the target antigen enables ELISAs to produce quantitative as well as qualitative data. And for the detection of popular analytes such as cytokines like interferon gamma from a range of sample types, kits are readily available, enabling detection of infections such as Bovine Tuberculosis (Figure 1).
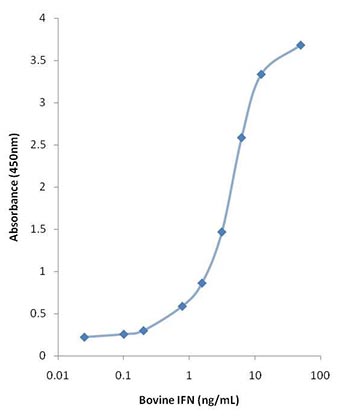
Fig.1. Typical assay curve using standards and reagents supplied in the Bovine IFN Gamma ELISA Kit (MCA5638KZZ).
There are different types of ELISAs, each with advantages and disadvantages depending on the analyte of interest and sample type being used. Selecting the type of ELISA to perform your experiment is probably the most critical part of planning and performing an ELISA.
There are four main types: direct, indirect, sandwich, and competitive. This blog is the first of a two-part series covering the different types of ELISA and their advantages and disadvantages. It is designed to help you determine which ELISA is right for you and hopefully prevent your ELISA assay from doing little!
Part One examines direct and indirect ELISAs in more detail, and Part Two covers sandwich and competitive ELISAs.
Direct ELISAs
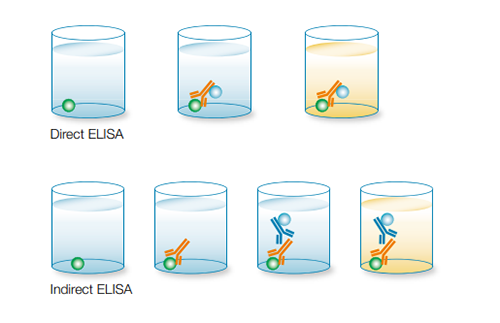
Direct ELISAs may be used to perform antibody screening and qualitative and quantitative detection of an analyte within a sample.
The first step of a direct ELISA is immobilization of the target analyte in your sample to the well of an ELISA plate by diluting the analyte in a coating buffer such as carbonate-bicarbonate buffer or phosphate buffered saline (PBS). An antibody targeting the analyte is then added to the well. In a direct ELISA, this antibody is conjugated to an enzyme, most commonly to horseradish peroxidase (HRP) and a substrate for the enzyme is then added to the well. The enzyme-substrate reaction results in a measurable change, which depending on the conjugate used, could be a colorimetric change, the emission of fluorescence, or a chemiluminescent signal.
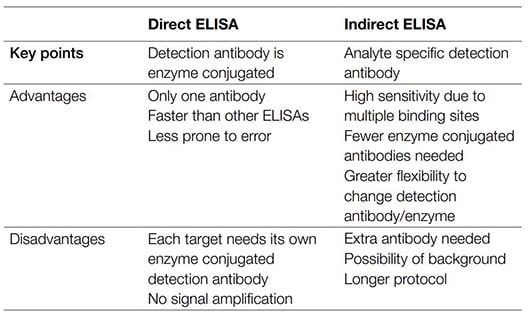
The main advantage of direct ELISA is that the antibody targeting the analyte of interest is directly conjugated, removing the need for the addition of a secondary antibody. This not only makes direct ELISA the quickest technique to perform but also reduces the potential for nonspecific background signal due to reagents used and human error. As fewer reagents and steps are required, direct ELISA tends to be less prone to errors. However, since all of the proteins in your sample, not just your protein of interest, are bound to the well, nonspecific binding and high background are more likely. Direct ELISAs also tend to be less sensitive than other types of ELISA based assays as there is no signal amplification. They also require a directly conjugated antibody specifically targeting your protein of interest, which might not be readily available. In addition, the direct labeling of a primary antibody can be expensive and time-consuming, and may reduce the ability of the antibody to bind to its target antigen. Direct ELISAs are typically used when an immune response needs to be analyzed, but an indirect ELISA can also be used to overcome some of the limitations of this application.
Indirect ELISAs
An indirect ELISA is similar to a direct ELISA in that both techniques require immobilization of the analyte to the ELISA plate as the first step. However, unlike direct ELISAs, detection is a two-step process. Rather than using a directly conjugated primary antibody, unconjugated primary antibodies are used and then detected using a labeled secondary antibody.
This use of a conjugated secondary antibody, rather than a directly conjugated primary antibody, results in amplification of signal from primary antibodies bound to the analyte, increasing the sensitivity compared to direct ELISA. The high level of sensitivity makes indirect ELISA the preferred method for quantifying total analyte concentration in a sample. However, the use of a secondary antibody does increase the possibility of background signal as the secondary antibody may cross-react with the adsorbed analyte. The additional step also means that an indirect ELISA takes longer to run than a direct ELISA.
Table 1. Advantages and disadvantages of direct and indirect ELISAs.
Learn More about ELISA with Bio-Rad’s Guide
Understand the principles of ELISA, find key facts, get troubleshooting advice, and learn how to optimize your ELISA to achieve successful results.
You may also be interested in...

View more Applications or ELISA blogs