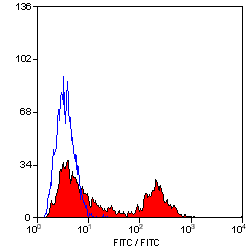
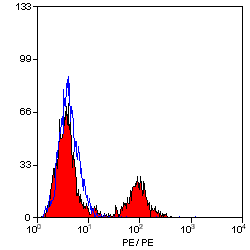
MHC Class II DQ DR Polymorphic antibody | 28.1
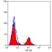

Mouse anti Sheep MHC Class II DQ DR Polymorphic:RPE
- Product Type
- Monoclonal Antibody
- Clone
- 28.1
- Isotype
- IgG1
- Specificity
- MHC Class II DQ DR Polymorphic
| Mouse anti Sheep MHC Class II DQ DR antibody, clone 28.1 recognizes a polymorphic epitope on ovine MHC class II DQ and DR molecules, which are constitutively expressed on antigen presenting cells such as dendritic cells, B lymphocytes, monocytes, macrophages, activated T lymphocytes and may be induced on a range of other cell types by interferon gamma. The major histocompatibility complex (MHC) is a cluster of genes some of which are important in the immune response to infections. In sheep, this complex is referred to as the ovine leukocyte antigen (OLA) region. There are 2 major types of MHC class IIa molecules encoded by the OLA which are DR and DQ each composed of an alpha and beta chain. Mouse anti Sheep MHC Class II DQ DR antibody, clone 28.1 recognizes ovine MHC II transfectants DQ - T28.1, DQ - T26.2 and DR - T31.3 but not DR - T8.1. (Ballingall, K. et al. 1995). |
- Target Species
- Sheep
- Species Cross-Reactivity
-
Target Species Cross Reactivity Bovine Goat - N.B. Antibody reactivity and working conditions may vary between species.
- Product Form
- Purified IgG conjugated to R. Phycoerythrin (RPE) - lyophilized
- Reconstitution
- Reconstitute with 1 ml distilled water
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
1% bovine serum albumin
5% sucrose - Immunogen
- Ovine alveolar macrophages.
- Fusion Partners
- Spleen cells from immunised BALB/c mice were fused with cells of the mouse NS1 myeloma cell line.
- Max Ex/Em
-
Fluorophore Excitation Max (nm) Emission Max (nm) RPE 488nm laser 496 578 - Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
This product should be stored undiluted.
DO NOT FREEZE.
This product is photosensitive and should be protected from light. Should this product contain a precipitate we recommend microcentrifugation before use.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | Neat | 1/10 |
- Flow Cytometry
- Use 10μl of the suggested working dilution to label 106 cells in 100μl
How to Use the Spectraviewer
Watch the Tool Tutorial Video ▸- Start by selecting the application you are interested in, with the option to select an instrument from the drop down menu or create a customized instrument
- Select the fluorophores or fluorescent proteins you want to include in your panel to check compatibility
- Select the lasers and filters you wish to include
- Select combined or multi-laser view to visualize the spectra
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Mouse IgG1 Negative Control:RPE | MCA928PE | F | 100 Tests | Log in | |||
| List Price | Your Price | ||||||
| Log in | |||||||
| Description | Mouse IgG1 Negative Control:RPE | ||||||
Source Reference
-
Puri, N.K. et al. (1985) Sheep lymphocyte antigens (OLA). II. Major histocompatibility complex class II molecules.
Immunology. 56 (4): 725-33.
References for MHC Class II DQ DR Polymorphic antibody
-
Puri, N.K. et al. (1987) Monoclonal antibodies to sheep MHC class II molecules recognize all HLA-D or subsets of HLA-D region products.
Hum Immunol. 20 (3): 195-207. -
Puri, N.K. & Brandon, M.R. (1987) Sheep MHC class II molecules. II. Identification and characterization of four distinct subsets of sheep MHC class II molecules.
Immunology. 62 (4): 575-80. -
Puri, N.K. et al. (1987) Sheep MHC class II molecules. I. Immunochemical characterization.
Immunology. 62 (4): 567-73. -
Puri, N.K. et al. (1987) Monoclonal antibodies to sheep MHC class I and class II molecules: biochemical characterization of three class I gene products and four distinct subpopulations of class II molecules.
Vet Immunol Immunopathol. 15 (1-2): 59-86. -
Ballingall. K. et al. (1995) Analysis of the fine specificities of sheep major histocompatibility complex class II - Specific monoclonal antibodies using mouse L - Cell transfectants.
Anim. Genet. 26: 79-84. -
Ferret-Bernard, S. et al. (2011) Mesenteric lymph node cells from neonates present a prominent IL-12 response to CpG oligodeoxynucleotide via an IL-15 feedback loop of amplification.
Vet Res. 42:19. -
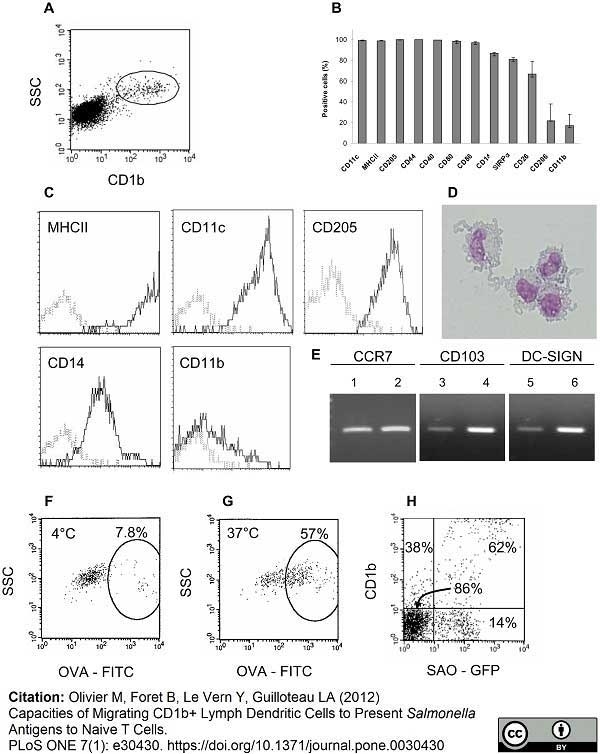
Olivier, M. et al. (2012) Capacities of Migrating CD1b Lymph Dendritic Cells to Present Salmonella Antigens to Naive T Cells.
PLoS One. 7: e30430. -
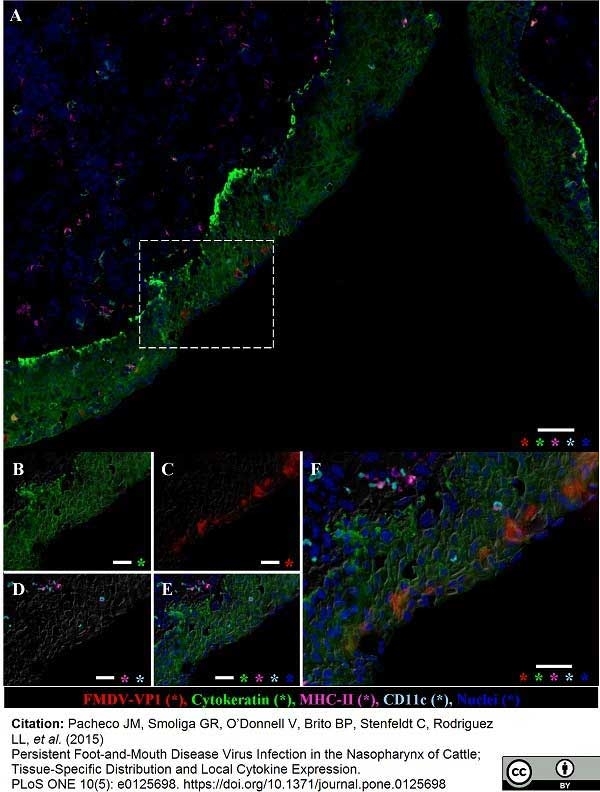
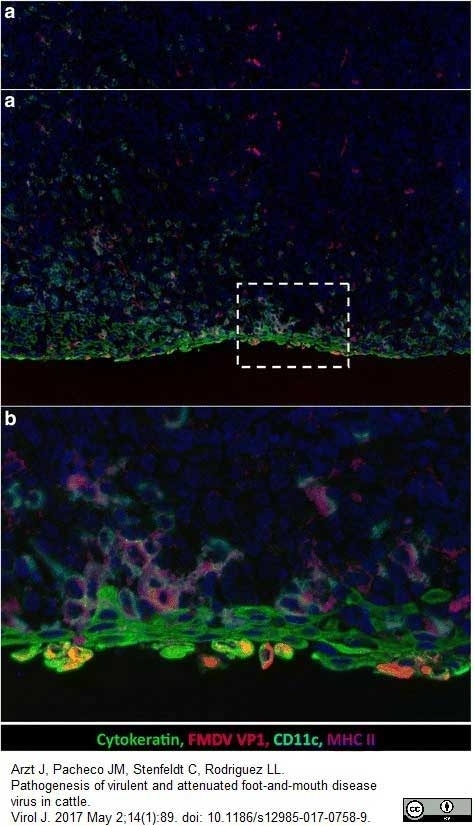
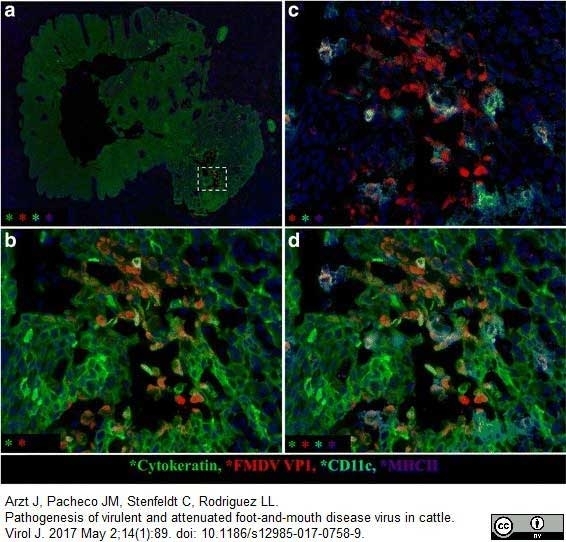
Arzt, J. et al. (2017) Pathogenesis of virulent and attenuated foot-and-mouth disease virus in cattle.
Virol J. 14 (1): 89.
View The Latest Product References
-
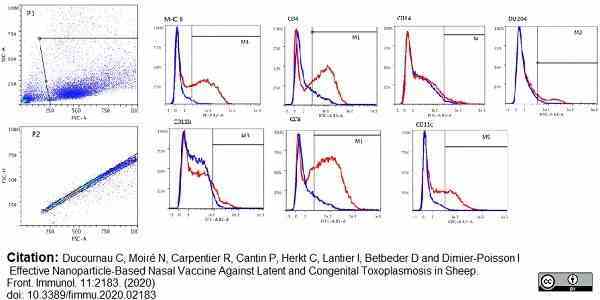
Ducournau, C. et al. (2020) Effective Nanoparticle-Based Nasal Vaccine Against Latent and Congenital Toxoplasmosis in Sheep.
Front Immunol. 11: 2183.
- RRID
- AB_324857
MCA2225PE
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Sheep ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up