Article: Generation and Characterization of Drug-Target Complex-Specific Antibodies
-
Monoclonal Generation
-
Custom Recombinant Monoclonal Antibody Generation
-
Webinars, Videos and Technical Articles
- Webinar: Overcome the Challenges of PK Assay Development Using TrailBlazer Antibodies
- Webinar: Generation of SARS-CoV-2 antibodies in multiple formats within four weeks
- Webinar: Recombinant Antibodies with SpyTag Technology
- Webinar: Transform bioanalytical assays with TrailBlazer Antibodies
- Webinar: Control your critical antibody reagents and avoid assay failure
- Webinar: Improve your antibody drug development assays
- Webinar: Optimize your assays using recombinant antibodies selected for desired affinity
- Webinar: The making of recombinant anti-idiotypic antibodies for high performance in bioanalytical assays
- Webinar: Human recombinant antibodies as positive controls and calibrators
- Webinar: How to overcome assay challenges using custom recombinant antibodies
- Webinar: Generation of high affinity recombinant antibodies for application in immuno-MRM
- Video: Generating anti-idiotypic antibodies for bioanalytical assays
- Video: Best practices for characterization and QC of anti-idiotypic antibodies for bioanalysis
- Video: Generation of drug-target complex specific antibodies
- Video: Antibodies for CAR-T cell therapy development
- Article: Monitoring antibody immune responses against biotherapeutic drugs
- Article: Effective tools for drug monitoring assays
- Article: An accelerated approach to sensitive ADA assays
- Article: Isolation of enzyme active site-specific recombinant antibodies by guided selection
- Article: Biomarker Assay Development using Highly Specific Recombinant Antibodies
- Article: Recombinant antibodies as standards for immunodiagnostic assays
- Article: High affinity antibodies for peptide enrichment immuno-MRM
- Article: Generation of antibodies against self-antigens
- Article: Generation and characterization of drug-target complex-specific antibodies
- Article: Antibodies for CAR-T Cell Therapy Development
- Poster: Faster Generation of Anti-Drug Antibodies Using SpyTag Technology
- Poster: CAR T cell analysis with modular antibodies
- Poster: Drug-Target-Complex Specific Antibodies for Pharmacokinetic Analysis of Biotherapeutics
- Poster: Characterization of anti-idiotypic antibodies for high performance in bioanalytical assays
- Poster: Recombinant anti-idiotypic antibodies for antibody drug development
- Poster: Generation of recombinant antibodies for Bio-Plex assays
-
Webinars, Videos and Technical Articles
-
Custom Recombinant Monoclonal Antibody Generation
s
Simplified sourcing via Scientist.com
s
Custom antibody project inquiry form
A personal, no obligation quotation for a custom monoclonal antibody generation project
s
Contact our custom antibody specialists
Tel: +49 (0) 89 80 90 95 45
Fax: +49 (0) 89 80 90 95 50
Office: Bio-Rad AbD Serotec GmbH, Campus Neuried, Anna-Sigmund-Str. 5, 82061 Neuried, Germany
Innovative Antibody Reagents for Bioanalysis of Biotherapeutics
HuCAL PLATINUM® technology was used to generate and characterize novel antibody entities that may be used to improve analysis of biotherapeutics during pre-clinical and clinical development, and during routine drug monitoring assessments in clinical practice.
Bio-Rad scientists generated antibody reagents capable of detecting the drug-target complex for five marketed therapeutic antibodies, with a high degree of sensitivity. Each of the reagents recognized the drug-target complex exclusively, with no affinity for free therapeutic antibody or unbound target molecules. HuCAL PLATINUM technology was also used to generate the first example of another specialized antibody that specifically recognized the monoclonal antibody drug in complex with its inhibitory anti-idiotypic antibody, but did not bind with the therapeutic or anti-idiotypic antibody alone.
These unique antibody reagents offer the opportunity for alternative assay formats, and overcome some of the challenges of sensitivity and selectivity faced by assay developers.
Therapeutic antibodies (also known as monoclonal antibody drugs), used to treat a growing number of serious diseases, may be present in several forms in human serum following administration; free antibody, partially bound antibody, or fully bound with the drug target. Ideally, analysis techniques to assess drug levels in the clinical stages of monoclonal drug development, or later for therapeutic drug monitoring, should be capable of measuring serum concentrations of the antibody in each of these forms when relevant.
Commonly used bridging assays for in vitro pharmacokinetic (PK) studies employ inhibitory or non-inhibitory anti-idiotypic antibody reagents designed to bind specifically to the human or humanized antibody drug and not to the excess of human immunoglobulin present in the patient sample (Figure 1). Bridging assays allow free drug or total drug to be detected within human serum samples. However, sensitivity may be limited as a low coating density of capture antibody is required to prevent the therapeutic antibody binding to the capture reagent with both arms. In addition, bridging assays do not work with monomeric drug antibodies such as antigen-binding fragments (Fabs).
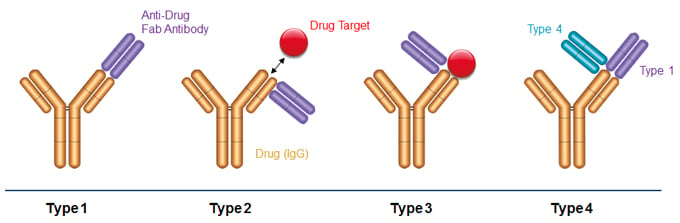
Fig. 1. Binding modes of anti-biotherapeutic antibodies. Type 1 anti-idiotype antibodies bind the paratope of the drug, inhibit drug target binding and detect free drug. Type 2 anti-idiotype antibodies bind outside the drug paratope, do not interfere with target binding, and detect total drug levels. Type 3 antibodies are specific for the drug-target complex and exclusively detect bound drug. Type 4 antibodies are specific for a complex formed between the drug and a Type 1 antibody.
To address these challenges, Harth et al. (2018) have generated antibody reagents that offer highly specific recognition of the drug-target complex for several approved therapeutic antibodies using the Human Combinatorial Antibody Libraries (HuCAL®) technology and CysDisplay®, a modified phage display method. These antibodies have been termed Type 3. They bind the drug-target complex exclusively and can be used in a PK antigen capture assay, providing an alternative to the bridging assay. This technology has also been used to develop a so-called Type 4 antibody that is specific for the drug when in complex with a Type 1 anti-idiotypic antibody, offering the possibility of additional assay design flexibility in cases where a Type 3 antibody cannot be made.
Therapeutic antibodies and antibody fragments represent a rapidly expanding class of biological medicine that have transformed the treatment of many long-term and complex diseases. These drugs offer excellent specificity, high affinity and potency, and extended in vivo half-life. The biosimilars market in this area is also expanding, where expiry of patent exclusivity has provided opportunities for highly similar versions of originator or reference drugs to enter the market.
Precise and consistent bioanalytical measurement of therapeutic antibodies and antibody fragments is critical for successful characterization and quantitative analysis during drug development, as well as for monitoring aspects of patient treatment during routine clinical practice. Ligand binding assays (LBAs) are performed on serum samples to determine the pharmacokinetic profile of a drug. LBAs must offer exceptionally high levels of specificity in order to bind the therapeutic antibody in the presence of a large excess of serum immunoglobulin. Inhibitory Type 1 anti-idiotypic antibodies are used to detect free drug, non-inhibitory Type 2 anti-idiotypic antibodies measure total drug levels (free, partially bound, and fully bound), Type 3 antibodies specifically recognize the drug-target complex, and Type 4 antibodies exclusively bind the drug-Type 1 antibody complex. Both Type 3 and Type 4 reagents allow detection of drug complexes without signal interference from free drug or target.
HuCAL PLATINUM Technology
HuCAL PLATINUM is an optimized, synthetic Fab library of 45 billion functional human antibody genes (Prassler et al. 2011). The library is based upon a modular framework of master genes with a diverse set of complementarity determining regions (CDRs) created by trinucleotide mutagenesis (TRIM). Structural diversity is provided by seven heavy chain and six light chain variable regions that combine to produce 42 antibody frameworks in the master library (Knappik et al. 2000; Prassler et al. 2011).
Screening of the library is performed in vitro, which allows guided selection of highly specific antibodies, such as antibodies that bind to a shared epitope between the drug and the target when in complex. Such specificity is very difficult to achieve using traditional monoclonal antibody generation methods that rely on the immunization of animals.
As the sequence of any selected antibody is known, it is possible to reproduce the genes synthetically if needed. Sequence availability secures antibody identity and recombinant production methods ensure a high level of consistency between batches. In addition, the original monovalent Fab antibody selected from the library can be easily converted to alternative formats, including bivalent mini-antibodies and full-length antibodies of different isotypes, offering flexibility for assay design. HuCAL is a well published and proven technology and has been used by the Bio-Rad Custom Antibody Team to generate antibodies for research and in vitro diagnostic applications since 2004.
Generation and Selection of Type 3 Antibodies
Bio-Rad scientists used the HuCAL PLATINUM antibody library in combination with CysDisplay, to generate drug-target complex-specific antibodies for five commercially available biotherapeutics: ranibizumab (Lucentis), adalimumab (Humira), golimumab (Simponi), trastuzumab (Herceptin) and omalizumab (Xolair).
Either solid phase (microtiter plate) or bead (magnetic bead) panning techniques were used to select Type 3 antibodies using immobilized drug-target complexes. To guide selection towards the drug-target complex alone and preferentially enrich clones with greater specificity, blocking was performed with isotype-matched control antibodies plus the target molecule (Figure 2). Three antibodies were also affinity matured by inserting pre-built trinucleotide libraries into the heavy chain CDR2 and/or the light chain CDR3. Selections were then carried out using increased stringency, and in all cases the affinity of the antibodies was improved while maintaining the specificity of the parental antibody. Some antibodies were also converted to a bivalent IgG1 format and produced in mammalian cells with the aim of increasing functional affinity, and consequently improving assay sensitivity when used as the detection reagent.
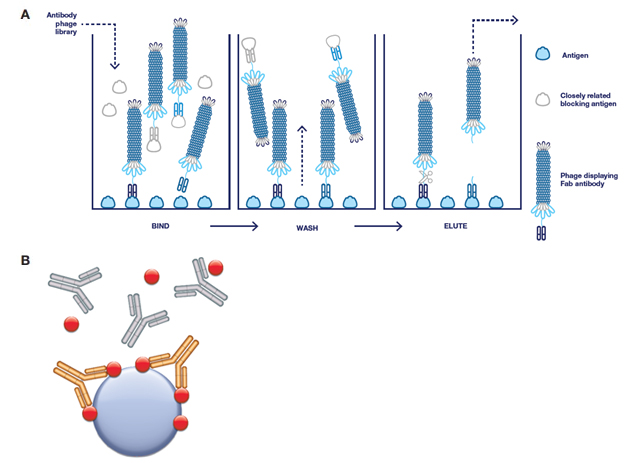
Fig. 2. Guided selection strategies for the generation of antibodies by phage display. A, overview of the procedure: addition of a closely related antigen blocks and removes phage-carrying antibodies that bind to that antigen (counter selection). B, Type 3 antibody selection: all selections were performed on the antibody drug (gold) in complex with its target (red) and immobilized. Blocking of the antibody phage library was conducted with isotype-matched control antibodies (gray), the drug target and human serum.
Detection of Drug-Target Complexes with Type 3 Antibodies
Antigen capture assays demonstrated that detection of drug-target complex with Type 3 antibodies was not influenced by matrix components in samples containing up to 90% normal human serum (NHS). Type 3 antibody AbD29928 directed against the ranibizumab/vascular endothelial growth factor (VEGF) complex displayed the same binding behavior when used at a range of concentrations regardless of NHS levels (Figure 3).
In antigen capture studies, Type 3 antibodies were able to detect both monovalent (Figure 3) and bivalent (Figure 4) therapeutic antibodies with comparable levels of sensitivity. Type 3 antibodies also demonstrated high levels of specificity for the drug-target complex and did not show binding affinity for the drug or the target alone, even at high drug concentrations (Figure 5).
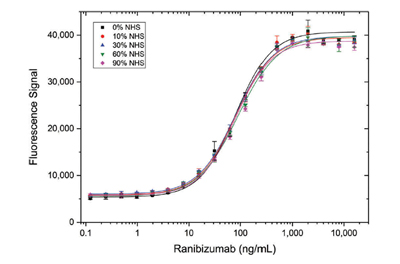
Fig. 3. Detection of ranibizumab (Fab antibody drug) in different serum concentrations using a Type 3 antibody. HRP conjugated Anti-Ranibizumab/VEGF Antibody (AbD29928, (HCA304) was mixed in various serum levels and titrated on immobilized ranibizumab in HISPEC Assay Diluent (BUF049), followed by signal detection using a fluorogenic peroxidase substrate.
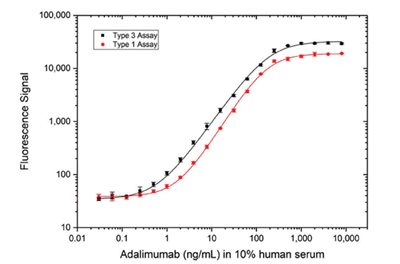
Fig. 4. Comparison of antigen capture and bridging assay formats. Detection of adalimumab using Type 3 Anti-Adalimumab/TNF Antibody (AbD18754, HCA206, KD 67 nM) in an antigen capture assay (black) or Type 1 capture and detection antibodies (AbD18654, HCA202, KD 0.15 nM; AbD18655_hIgG1, HCA204, KD 0.06 nM) in a bridging assay (red), demonstrating that equivalent high sensitivity can be achieved with both assay formats, despite the type 3 antibody having a much lower affinity than both the type 1 antibodies.
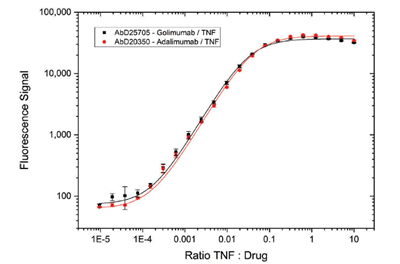
Fig. 5. Immunocapture of drug/TNF complexes by Type 3 antibodies Anti-Golimumab/TNF Antibody (AbD25705, HCA274) and Anti-Adalimumab/TNF Antibody (AbD20350, HCA231). Type 3 antibodies in Fab format were coated on a microtiter plate. Drug-target complexes at different ratios were added. Detection was performed with Anti-Human IgG (Fc) CH2 Domain:HRP Antibody (MCA647P) specific for golimumab or adalimumab.
Novel Type 4 Antibodies
In a proof-of-concept experiment, Bio-Rad scientists were able to isolate a Type 4 antibody (AbD23820) by panning on the drug trastuzumab bound to the high-affinity Type 1 antibody AbD18018 in the IgG1 format. Specificity titration ELISA results demonstrated that AbD23820 was able to bind the drug-Type 1 antibody complex, but did not bind to free trastuzumab or Type 1 antibody (AbD18018) alone (Figure 6).
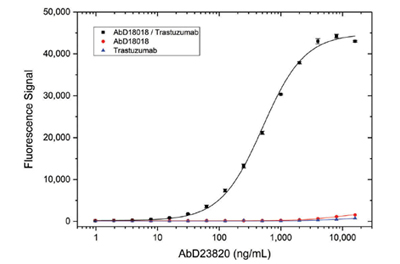
Fig. 6. Type 4 antibody specificity titration ELISA. Type 1 Anti-Trastuzumab Antibody AbD18018 in IgG1 format (HCA177) or trastuzumab were coated on a microtiter plate. The trastuzumab/Type 1 complex was formed by adding trastuzumab to the wells coated with the Type 1 antibody. After washing, Type 4 antibody AbD23820 specific for the trastuzumab/Type 1 complex was titrated in PBST and added. Detection was performed using an HRP conjugated anti-Strep-tag antibody.
Conclusions
Type 3 antibodies generated using the HuCAL PLATINUM technology provide exceptional levels of specificity for drug-target complexes associated with the approved therapeutic antibodies ranibizumab, adalimumab, golimumab, trastuzumab, and omalizumab. For PK studies, Type 3 antibodies offer the assay developer a robust and simpler alternative to conventional bridging assays, which is essential when the therapeutic antibody is in monovalent format. Antigen capture assays using Type 3 antibody reagents offer the same high level of sensitivity for detection of both monovalent and bivalent therapeutic antibodies.
Type 4 antibodies, generated using the same technology platform, enable detection of the complex formed between the therapeutic antibody and Type 1 antibody. Type 4 reagents may prove useful in cases where the drug target is not easily available (or very costly) and/or when it is not possible to generate Type 3 antibodies against the drug-target complex.
Alongside Type 1 and Type 2 antibody reagents, Type 3 and Type 4 antibodies provide extremely valuable additions to the LBA toolbox and may enable the development of more sophisticated analysis tools to support the rapidly evolving area of biotherapeutics.
References:
- Harth S et al. (2019). Generation by phage display and characterization of drug-target complex-specific antibodies for pharmacokinetic analysis of biotherapeutics. mAbs 11,1:178-90.
- Knappik A et al. (2000). Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol, 296, 57-86.
- Prassler J et al. (2011). HuCAL PLATINUM, a synthetic Fab library optimized for sequence diversity and superior performance in mammalian expression systems. J Mol Biol, 413, 261-278.






